The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia
- PMID: 22761937
- PMCID: PMC3384594
- DOI: 10.1371/journal.pone.0039959
The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia
Abstract
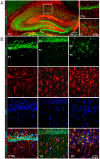
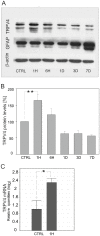
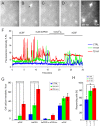
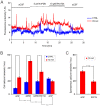
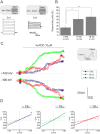
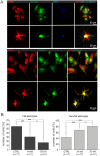
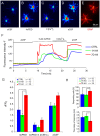
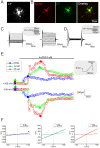
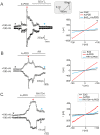
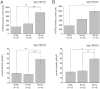
The polymodal transient receptor potential vanilloid 4 (TRPV4) channel, a member of the TRP channel family, is a calcium-permeable cationic channel that is gated by various stimuli such as cell swelling, low pH and high temperature. Therefore, TRPV4-mediated calcium entry may be involved in neuronal and glia pathophysiology associated with various disorders of the central nervous system, such as ischemia. The TRPV4 channel has been recently found in adult rat cortical and hippocampal astrocytes; however, its role in astrocyte pathophysiology is still not defined. In the present study, we examined the impact of cerebral hypoxia/ischemia (H/I) on the functional expression of astrocytic TRPV4 channels in the adult rat hippocampal CA1 region employing immunohistochemical analyses, the patch-clamp technique and microfluorimetric intracellular calcium imaging on astrocytes in slices as well as on those isolated from sham-operated or ischemic hippocampi. Hypoxia/ischemia was induced by a bilateral 15-minute occlusion of the common carotids combined with hypoxic conditions. Our immunohistochemical analyses revealed that 7 days after H/I, the expression of TRPV4 is markedly enhanced in hippocampal astrocytes of the CA1 region and that the increasing TRPV4 expression coincides with the development of astrogliosis. Additionally, adult hippocampal astrocytes in slices or cultured hippocampal astrocytes respond to the TRPV4 activator 4-alpha-phorbol-12,-13-didecanoate (4αPDD) by an increase in intracellular calcium and the activation of a cationic current, both of which are abolished by the removal of extracellular calcium or exposure to TRP antagonists, such as Ruthenium Red or RN1734. Following hypoxic/ischemic injury, the responses of astrocytes to 4αPDD are significantly augmented. Collectively, we show that TRPV4 channels are involved in ischemia-induced calcium entry in reactive astrocytes and thus, might participate in the pathogenic mechanisms of astroglial reactivity following ischemic insult.
Conflict of interest statement
Figures

References
-
- Chvatal A, Anderova M, Neprasova H, Prajerova I, Benesova J, et al. Pathological potential of astroglia. Physiol Res. 2008;57:S101–110. - PubMed
-
- Verkhratsky A, Anderova M, Chvatal A. Differential calcium signalling in neuronal-glial networks. Front Biosci. 2009;14:2004–2016. - PubMed
-
- Nedergaard M, Rodriguez JJ, Verkhratsky A. Glial calcium and diseases of the nervous system. Cell Calcium. 2010;47:140–149. - PubMed
-
- Latour I, Hamid J, Beedle AM, Zamponi GW, Macvicar BA. Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia. 2003;41:347–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

