Vasopressin V2R-targeting peptide carrier mediates siRNA delivery into collecting duct cells
- PMID: 22761946
- PMCID: PMC3386242
- DOI: 10.1371/journal.pone.0040010
Vasopressin V2R-targeting peptide carrier mediates siRNA delivery into collecting duct cells
Abstract
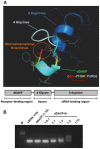
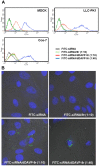
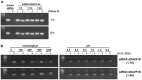
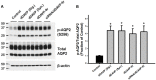
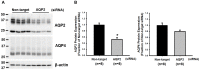
Internalization of receptor proteins after interacting with specific ligands has been proposed to facilitate siRNA delivery into the target cells via receptor-mediated siRNA transduction. In this study, we demonstrated a novel method of vasopressin V2 receptor (V2R)-mediated siRNA delivery against AQP2 in primary cultured inner medullary collecting duct (IMCD) cells of rat kidney. We synthesized the dDAVP conjugated with nine D-arginines (dDAVP-9r) as a peptide carrier for siRNA delivery. The structure of synthetic peptide carrier showed two regions (i.e., ligand domain to V2R (dDAVP) and siRNA carrying domain (nine D-arginine)) bisected with a spacer of four glycines. The results revealed that 1) synthesized dDAVP-9r peptides formed a stable polyplex with siRNA; 2) siRNA/dDAVP-9r polyplex could bind to the V2R of IMCD cells and induced AQP2 phosphorylation (Ser 256); 3) siRNA/dDAVP-9r polyplex was stable in response to the wide range of different osmolalities, pH levels, or to the RNases; 4) fluorescein-labeled siRNA was delivered into V2R-expressing MDCK and LLC-PK1 cells by siRNA/dDAVP-9r polyplex, but not into the V2R-negative Cos-7 cells; and 5) AQP2-siRNA/dDAVP-9r polyplex effectively delivered siRNA into the IMCD cells, resulting in the significant decrease of protein abundance of AQP2, but not AQP4. Therefore, for the first time to our knowledge, we demonstrated that V2R-mediated siRNA delivery could be exploited to deliver specific siRNA to regulate abnormal expression of target proteins in V2R-expressing kidney cells. The methods could be potentially used in vivo to regulate abnormal expression of proteins associated with disease conditions in the V2R-expressing kidney cells.
Conflict of interest statement
Figures
Similar articles
-
Extracellular pH affects phosphorylation and intracellular trafficking of AQP2 in inner medullary collecting duct cells.Am J Physiol Renal Physiol. 2015 Apr 1;308(7):F737-48. doi: 10.1152/ajprenal.00376.2014. Epub 2015 Jan 28. Am J Physiol Renal Physiol. 2015. PMID: 25651562
-
Lixivaptan, a New Generation Diuretic, Counteracts Vasopressin-Induced Aquaporin-2 Trafficking and Function in Renal Collecting Duct Cells.Int J Mol Sci. 2019 Dec 26;21(1):183. doi: 10.3390/ijms21010183. Int J Mol Sci. 2019. PMID: 31888044 Free PMC article.
-
Psychotropic drugs upregulate aquaporin-2 via vasopressin-2 receptor/cAMP/protein kinase A signaling in inner medullary collecting duct cells.Am J Physiol Renal Physiol. 2021 May 1;320(5):F963-F971. doi: 10.1152/ajprenal.00576.2020. Epub 2021 Apr 12. Am J Physiol Renal Physiol. 2021. PMID: 33843270
-
Aquaporin-2 membrane targeting: still a conundrum.Am J Physiol Renal Physiol. 2017 Apr 1;312(4):F744-F747. doi: 10.1152/ajprenal.00010.2017. Epub 2017 Feb 8. Am J Physiol Renal Physiol. 2017. PMID: 28179252 Review.
-
The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors.Am J Physiol Renal Physiol. 2014 May 1;306(9):F931-40. doi: 10.1152/ajprenal.00604.2013. Epub 2014 Mar 5. Am J Physiol Renal Physiol. 2014. PMID: 24598801 Review.
Cited by
-
Nano-Technological Approaches for Targeting Kidney Diseases With Focus on Diabetic Nephropathy: Recent Progress, and Future Perspectives.Front Bioeng Biotechnol. 2022 May 13;10:870049. doi: 10.3389/fbioe.2022.870049. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35646840 Free PMC article. Review.
-
Design of a multicomponent peptide-woven nanocomplex for delivery of siRNA.PLoS One. 2015 Feb 23;10(2):e0118310. doi: 10.1371/journal.pone.0118310. eCollection 2015. PLoS One. 2015. PMID: 25705892 Free PMC article.
-
Targeting and therapeutic peptide-based strategies for polycystic kidney disease.Adv Drug Deliv Rev. 2020;161-162:176-189. doi: 10.1016/j.addr.2020.08.011. Epub 2020 Aug 29. Adv Drug Deliv Rev. 2020. PMID: 32866560 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

