Expression and cellular immunogenicity of a transgenic antigen driven by endogenous poxviral early promoters at their authentic loci in MVA
- PMID: 22761956
- PMCID: PMC3384612
- DOI: 10.1371/journal.pone.0040167
Expression and cellular immunogenicity of a transgenic antigen driven by endogenous poxviral early promoters at their authentic loci in MVA
Abstract
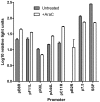
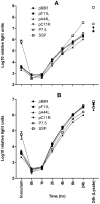
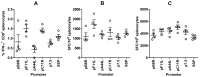
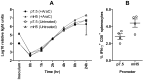
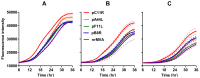
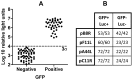
CD8(+) T cell responses to vaccinia virus are directed almost exclusively against early gene products. The attenuated strain modified vaccinia virus Ankara (MVA) is under evaluation in clinical trials of new vaccines designed to elicit cellular immune responses against pathogens including Plasmodium spp., M. tuberculosis and HIV-1. All of these recombinant MVAs (rMVA) utilize the well-established method of linking the gene of interest to a cloned poxviral promoter prior to insertion into the viral genome at a suitable locus by homologous recombination in infected cells. Using BAC recombineering, we show that potent early promoters that drive expression of non-functional or non-essential MVA open reading frames (ORFs) can be harnessed for immunogenic expression of recombinant antigen. Precise replacement of the MVA orthologs of C11R, F11L, A44L and B8R with a model antigen positioned to use the same translation initiation codon allowed early transgene expression similar to or slightly greater than that achieved by the commonly-used p7.5 or short synthetic promoters. The frequency of antigen-specific CD8(+) T cells induced in mice by single shot or adenovirus-prime, rMVA-boost vaccination were similarly equal or marginally enhanced using endogenous promoters at their authentic genomic loci compared to the traditional constructs. The enhancement in immunogenicity observed using the C11R or F11L promoters compared with p7.5 was similar to that obtained with the mH5 promoter compared with p7.5. Furthermore, the growth rates of the viruses were unimpaired and the insertions were genetically stable. Insertion of a transgenic ORF in place of a viral ORF by BAC recombineering can thus provide not only a potent promoter, but also, concomitantly, a suitable insertion site, potentially facilitating development of MVA vaccines expressing multiple recombinant antigens.
Conflict of interest statement
Figures

References
-
- Smith GL, Mackett M, Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983;302:490–495. - PubMed
-
- Paoletti E, Lipinskas BR, Samsonoff C, Mercer S, Panicali D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1984;81:193–197. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

