Intragastric and Intranasal Administration of Lactobacillus paracasei NCC2461 Modulates Allergic Airway Inflammation in Mice
- PMID: 22762009
- PMCID: PMC3382844
- DOI: 10.1155/2012/686739
Intragastric and Intranasal Administration of Lactobacillus paracasei NCC2461 Modulates Allergic Airway Inflammation in Mice
Abstract
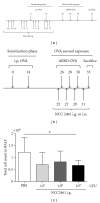
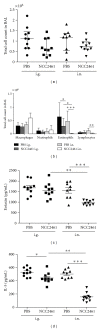
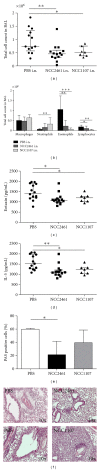
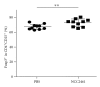
Introduction. Preclinical and clinical evidences for a role of oral probiotics in the management of allergic diseases are emerging. Aim. We aimed at testing the immunomodulatory effects of intranasal versus intragastric administration of Lactobacillus paracasei NCC2461 in a mouse model of allergic airway inflammation and the specificity of different probiotics by comparing L. paracasei NCC2461 to Lactobacillus plantarum NCC1107. Methods. L. paracasei NCC2461 or L. plantarum NCC1107 strains were administered either intragastrically (NCC2461) or intranasally (NCC2461 or NCC1107) to OVA-sensitized mice challenged with OVA aerosols. Inflammatory cell recruitment into BALF, eotaxin and IL-5 production in the lungs were measured. Results. Intranasal L. paracasei NCC2461 efficiently protected sensitized mice upon exposure to OVA aerosols in a dose-dependent manner as compared to control mice. Inflammatory cell number, eotaxin and IL-5 were significantly reduced in BALF. Intranasal supplementation of L. paracasei NCC2461 was more potent than intragastric application in limiting the allergic response and possibly linked to an increase in T regulatory cells in the lungs. Finally, intranasal L. plantarum NCC1107 reduced total and eosinophilic lung inflammation, but increased neutrophilia and macrophages infiltration. Conclusion. A concerted selection of intervention schedule, doses, and administration routes (intranasal versus intragastric) may markedly contribute to modulate airway inflammation in a probiotic strain-specific manner.
Figures
Similar articles
-
Effect of Lactobacillus paracasei NCC2461 on antigen-specific T-cell mediated immune responses in aged mice.Rejuvenation Res. 2008 Oct;11(5):957-64. doi: 10.1089/rej.2008.0780. Rejuvenation Res. 2008. PMID: 18922048
-
High-fat diet-induced obesity is attenuated by probiotic strain Lactobacillus paracasei ST11 (NCC2461) in rats.Obes Res Clin Pract. 2008 Sep;2(3):I-II. doi: 10.1016/j.orcp.2008.04.003. Obes Res Clin Pract. 2008. PMID: 24351773
-
Comparison of two oral probiotic preparations in a randomized crossover trial highlights a potentially beneficial effect of Lactobacillus paracasei NCC2461 in patients with allergic rhinitis.Clin Transl Allergy. 2014 Jan 6;4(1):1. doi: 10.1186/2045-7022-4-1. Clin Transl Allergy. 2014. PMID: 24393277 Free PMC article.
-
Lactobacillus paracasei ATG-E1 improves particulate matter 10 plus diesel exhaust particles (PM10D)-induced airway inflammation by regulating immune responses.Front Microbiol. 2023 Apr 27;14:1145546. doi: 10.3389/fmicb.2023.1145546. eCollection 2023. Front Microbiol. 2023. PMID: 37180255 Free PMC article.
-
Immune modulation property of Lactobacillus paracasei NCC2461 (ST11) strain and impact on skin defences.Benef Microbes. 2014 Jun 1;5(2):129-36. doi: 10.3920/BM2013.0014. Benef Microbes. 2014. PMID: 24322880 Review.
Cited by
-
Possible modulating functions of probiotic Lactiplantibacillus plantarum in particulate matter-associated pulmonary inflammation.Front Cell Infect Microbiol. 2024 Jan 9;13:1290914. doi: 10.3389/fcimb.2023.1290914. eCollection 2023. Front Cell Infect Microbiol. 2024. PMID: 38264731 Free PMC article. Review.
-
Lung Microbiome in Asthma: Current Perspectives.J Clin Med. 2019 Nov 14;8(11):1967. doi: 10.3390/jcm8111967. J Clin Med. 2019. PMID: 31739446 Free PMC article. Review.
-
Eosinophilic Esophagitis and Microbiota: State of the Art.Front Immunol. 2021 Feb 19;12:595762. doi: 10.3389/fimmu.2021.595762. eCollection 2021. Front Immunol. 2021. PMID: 33679739 Free PMC article. Review.
-
The Future of Bronchopulmonary Dysplasia: Emerging Pathophysiological Concepts and Potential New Avenues of Treatment.Front Med (Lausanne). 2017 May 22;4:61. doi: 10.3389/fmed.2017.00061. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28589122 Free PMC article. Review.
-
Can probiotics be used in the prevention and treatment of bronchial asthma?Pharmacol Rep. 2024 Aug;76(4):740-753. doi: 10.1007/s43440-024-00618-0. Epub 2024 Jul 1. Pharmacol Rep. 2024. PMID: 38951480 Free PMC article. Review.
References
-
- Guarner F, Schaafsma GJ. Probiotics. International Journal of Food Microbiology. 1998;39(3):237–238. - PubMed
-
- Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. Journal of Allergy and Clinical Immunology. 2006;117(5):969–977. - PubMed
-
- Guarner F, Requena T, Marcos A. Consensus statements from the workshop "Probiotics and health: Scientific evidence". Nutricion Hospitalaria. 2010;25(5):700–704. - PubMed
-
- Kalliomäki M, Antoine JM, Herz U, Rijkers GT, Wells JM, Mercenier A. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of allergic diseases by probiotics. Journal of Nutrition. 2010;140(3):713S–721S. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

