Physiological phenotype and vulnerability in Parkinson's disease
- PMID: 22762023
- PMCID: PMC3385938
- DOI: 10.1101/cshperspect.a009290
Physiological phenotype and vulnerability in Parkinson's disease
Abstract
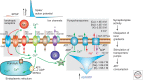
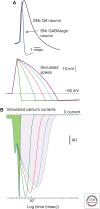
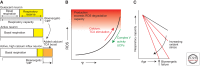
This review will focus on the principles underlying the hypothesis that neuronal physiological phenotype-how a neuron generates and regulates action potentials-makes a significant contribution to its vulnerability in Parkinson's disease (PD) and aging. A cornerstone of this hypothesis is that the maintenance of ionic gradients underlying excitability can pose a significant energetic burden for neurons, particularly those that have sustained residence times at depolarized membrane potentials, broad action potentials, prominent Ca(2+) entry, and modest intrinsic Ca(2+) buffering capacity. This energetic burden is shouldered in neurons primarily by mitochondria, the sites of cellular respiration. Mitochondrial respiration increases the production of damaging superoxide and other reactive oxygen species (ROS) that have widely been postulated to contribute to cellular aging and PD. Many of the genetic mutations and toxins associated with PD compromise mitochondrial function, providing a mechanistic linkage between known risk factors and cellular physiology that could explain the pattern of pathology in PD. Because much of the mitochondrial burden created by this at-risk phenotype is created by Ca(2+) entry through L-type voltage-dependent channels for which there are antagonists approved for human use, a neuroprotective strategy to reduce this burden is feasible.
Figures

Similar articles
-
Calcium, bioenergetics, and neuronal vulnerability in Parkinson's disease.J Biol Chem. 2013 Apr 12;288(15):10736-41. doi: 10.1074/jbc.R112.410530. Epub 2012 Oct 19. J Biol Chem. 2013. PMID: 23086948 Free PMC article. Review.
-
What causes the death of dopaminergic neurons in Parkinson's disease?Prog Brain Res. 2010;183:59-77. doi: 10.1016/S0079-6123(10)83004-3. Prog Brain Res. 2010. PMID: 20696315
-
The origins of oxidant stress in Parkinson's disease and therapeutic strategies.Antioxid Redox Signal. 2011 Apr 1;14(7):1289-301. doi: 10.1089/ars.2010.3521. Epub 2010 Dec 15. Antioxid Redox Signal. 2011. PMID: 20712409 Free PMC article. Review.
-
A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson's disease.Mov Disord. 2010;25 Suppl 1:S63-70. doi: 10.1002/mds.22801. Mov Disord. 2010. PMID: 20187241
-
The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson's disease.Gene. 2013 Dec 10;532(1):18-23. doi: 10.1016/j.gene.2013.07.085. Epub 2013 Aug 15. Gene. 2013. PMID: 23954870 Review.
Cited by
-
Mitochondrial oxidant stress in locus coeruleus is regulated by activity and nitric oxide synthase.Nat Neurosci. 2014 Jun;17(6):832-40. doi: 10.1038/nn.3717. Epub 2014 May 11. Nat Neurosci. 2014. PMID: 24816140 Free PMC article.
-
RISC in PD: the impact of microRNAs in Parkinson's disease cellular and molecular pathogenesis.Front Mol Neurosci. 2013 Nov 20;6:40. doi: 10.3389/fnmol.2013.00040. Front Mol Neurosci. 2013. PMID: 24312000 Free PMC article. Review.
-
Levodopa treatment: impacts and mechanisms throughout Parkinson's disease progression.J Neural Transm (Vienna). 2025 Jun;132(6):743-779. doi: 10.1007/s00702-025-02893-4. Epub 2025 Apr 11. J Neural Transm (Vienna). 2025. PMID: 40214767 Free PMC article. Review.
-
Mechanisms of protein toxicity in neurodegenerative diseases.Cell Mol Life Sci. 2018 Sep;75(17):3159-3180. doi: 10.1007/s00018-018-2854-4. Epub 2018 Jun 12. Cell Mol Life Sci. 2018. PMID: 29947927 Free PMC article. Review.
-
Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):156-178. doi: 10.1111/jnc.13572. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26865375 Free PMC article. Review.
References
-
- Aguilaniu H, Durieux J, Dillin A 2005. Metabolism, ubiquinone synthesis, and longevity. Genes Dev 19: 2399–2406 - PubMed
-
- Aston-Jones G 2005. Brain structures and receptors involved in alertness. Sleep Med 6: S3–S7 - PubMed
-
- Augustine GJ, Santamaria F, Tanaka K 2003. Local calcium signaling in neurons. Neuron 40: 331–346 - PubMed
-
- Bardo S, Cavazzini MG, Emptage N 2006. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci 27: 78–84 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
