Glycyrrhizin in patients who failed previous interferon alpha-based therapies: biochemical and histological effects after 52 weeks
- PMID: 22762137
- PMCID: PMC3584517
- DOI: 10.1111/j.1365-2893.2011.01579.x
Glycyrrhizin in patients who failed previous interferon alpha-based therapies: biochemical and histological effects after 52 weeks
Abstract
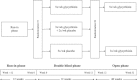
Chronic hepatitis C patients often fail to respond to interferon-based therapies. This phase III study aimed at confirming the efficacy and safety of glycyrrhizin in interferon + ribavirin-based therapy non-responders. A randomised, double-blind, placebo-controlled, comparison of glycyrrhizin, administered intravenously 5×/or 3×/week, and 5×/week placebo for 12 weeks to 379 patients, was followed by a randomised, open comparison of glycyrrhizin i.v. 5×/versus 3×/week for 40 weeks. Primary endpoints were: (1) the proportion of patients with ≥50% ALT (alanine aminotransferase) reduction after 12 weeks double-blind phase, and (2) the proportion of patients with improvement of necro-inflammation after 52 weeks as compared with baseline. The proportion of patients with ALT reduction ≥50% after 12 weeks was significantly higher with 5×/week glycyrrhizin (28.7%, P < 0.0001) and 3×/week glycyrrhizin (29.0%, P < 0.0001) compared with placebo (7.0%). The proportion of patients with improvement in necro-inflammation after 52 weeks was 44.9% with 5×/week and 46.0% with 3×/week, respectively. Glycyrrhizin exhibited a significantly higher ALT reduction compared to placebo after 12 weeks of therapy and an improvement of necro-inflammation and fibrosis after 52-weeks treatment. Generally, glycyrrhizin treatment was well tolerated.
© 2012 Blackwell Publishing Ltd.
Figures


References
-
- Craxi A. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol. 2011;55(2):245–264. - PubMed
-
- Di Bisceglie AM, Goodman ZD, Ishak KG, Hoofnagle JH, Melpolder JJ, Alter HJ. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology. 1991;14:969–974. - PubMed
-
- Bruix J, Poynard T, Colombo M, et al. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140(7):1990–1999. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

