Transglutaminase 2 cross-linking activity is linked to invadopodia formation and cartilage breakdown in arthritis
- PMID: 22762273
- PMCID: PMC3580551
- DOI: 10.1186/ar3899
Transglutaminase 2 cross-linking activity is linked to invadopodia formation and cartilage breakdown in arthritis
Abstract
Introduction: The microenvironment surrounding inflamed synovium leads to the activation of fibroblast-like synoviocytes (FLSs), which are important contributors to cartilage destruction in rheumatoid arthritic (RA) joints. Transglutaminase 2 (TG2), an enzyme involved in extracellular matrix (ECM) cross-linking and remodeling, is activated by inflammatory signals. This study was undertaken to assess the potential contribution of TG2 to FLS-induced cartilage degradation.
Methods: Transglutaminase (TGase) activity and collagen degradation were assessed with the immunohistochemistry of control, collagen-induced arthritic (CIA) or TG2 knockdown (shRNA)-treated joint tissues. TGase activity in control (C-FLS) and arthritic (A-FLS) rat FLSs was measured by in situ 5-(biotinamido)-pentylamine incorporation. Invadopodia formation and functions were measured in rat FLSs and cells from normal (control; C-FLS) and RA patients (RA-FLS) by in situ ECM degradation. Immunoblotting, enzyme-linked immunosorbent assay (ELISA), and p3TP-Lux reporter assays were used to assess transforming growth factor-β (TGF-β) production and activation.
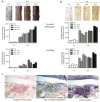
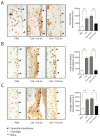
Results: TG2 and TGase activity were associated with cartilage degradation in CIA joints. In contrast, TGase activity and cartilage degradation were reduced in joints by TG2 knockdown. A-FLSs displayed higher TGase activity and TG2 expression in ECM than did C-FLSs. TG2 knockdown or TGase inhibition resulted in reduced invadopodia formation in rat and human arthritic FLSs. In contrast, increased invadopodia formation was noted in response to TGase activity induced by TGF-β, dithiothreitol (DTT), or TG2 overexpression. TG2-induced increases in invadopodia formation were blocked by TGF-β neutralization or inhibition of TGF-βR1.
Conclusions: TG2, through its TGase activity, is required for ECM degradation in arthritic FLS and CIA joints. Our findings provide a potential target to prevent cartilage degradation in RA.
Figures



References
-
- Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Immunol. 2001;167:5381–5385. - PubMed
-
- Garcia-Vicuna R, Gomez-Gaviro MV, Dominguez-Luis MJ, Pec MK, Gonzalez-Alvaro I, Alvaro-Gracia JM, Diaz-Gonzalez F. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 2004;50:3866–3877. doi: 10.1002/art.20615. - DOI - PubMed
-
- Remmers EF, Lafyatis R, Kumkumian GK, Case JP, Roberts AB, Sporn MB, Wilder RL. Cytokines and growth regulation of synoviocytes from patients with rheumatoid arthritis and rats with streptococcal cell wall arthritis. Growth Factors. 1990;2:179–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

