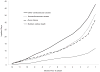
In-hospital resource use and medical costs in the last year of life by mode of death (from the HF-ACTION randomized controlled trial)
- PMID: 22762718
- PMCID: PMC3462294
- DOI: 10.1016/j.amjcard.2012.05.059
In-hospital resource use and medical costs in the last year of life by mode of death (from the HF-ACTION randomized controlled trial)
Abstract
Patterns of medical resource use near the end of life may differ across modes of death. The aim of this study was to characterize patterns of inpatient resource use and direct costs for patients with heart failure (HF) who died of sudden cardiac death (SCD), HF, other cardiovascular causes, or noncardiovascular causes during the last year of life. Data were from a randomized trial of exercise training in patients with HF. Mode of death was adjudicated by an end point committee. Generalized estimating equations were used to compare hospitalizations, inpatient days, and inpatient costs incurred during the final year of life in patients who died of different causes, adjusting for clinical and treatment characteristics. Of 2,331 patients enrolled in the trial, 231 died after ≥1 year of follow-up with an adjudicated mode of death, including 72 of SCD, 80 of HF, 34 of other cardiovascular causes, and 45 of noncardiovascular causes. Patients who died of SCD were younger, had less severe HF, and incurred fewer hospitalizations, fewer inpatient days, and lower inpatient costs than patients who died of other causes. After adjustment for patient characteristics, inpatient resource use varied by 2 to 4 times across modes of death, suggesting that cost-effectiveness analyses of interventions that reduce mortality from SCD compared to other causes should incorporate mode-specific end-of-life costs. In conclusion, resource use and associated medical costs in the last year of life differed markedly in patients with HF who experienced SCD and patients who died of other causes.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
References
-
- Whellan DJ, O’Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston-Miller N, Fleg JL, Schulman KA Piña IL; HF-ACTION Trial Investigators. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–211. - PubMed
-
- O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F Piña IL; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. - PMC - PubMed
-
- Reed SD, Whellan DJ, Li Y, Friedman JY, Stephen J, Ellis SJ, Piña IL, Settles SJ, Davidson-Ray L, Johnson JL, Cooper LS, O’Connor CM, Schulman KA. Economic evaluation of the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) randomized controlled trial: an exercise training study of patients with chronic heart failure. Circ Cardiovasc Qual Outcomes. 2010;3:374–381. - PMC - PubMed
-
- Unroe KT, Greiner MA, Hernandez AF, Whellan DJ, Kaul P, Schulman KA, Peterson ED, Curtis LH. Resource use in the last 6 months of life among medicare beneficiaries with heart failure, 2000–2007. Arch Intern Med. 2011;171:196–203. - PubMed
-
- Connor SR, Elwert F, Spence C, Christakis NA. Racial disparity in hospice use in the United States in 2002. Palliat Med. 2008;22:205–213. - PubMed
Publication types
MeSH terms
Grants and funding
- 5U01HL066461/HL/NHLBI NIH HHS/United States
- U01 HL064250/HL/NHLBI NIH HHS/United States
- R37 AG018915/AG/NIA NIH HHS/United States
- U01 HL064265/HL/NHLBI NIH HHS/United States
- U01 HL066497/HL/NHLBI NIH HHS/United States
- U01 HL064264/HL/NHLBI NIH HHS/United States
- 5U01HL066482/HL/NHLBI NIH HHS/United States
- P60AG010484/AG/NIA NIH HHS/United States
- U01 HL068980/HL/NHLBI NIH HHS/United States
- 5U01HL064250/HL/NHLBI NIH HHS/United States
- 5U01HL064265/HL/NHLBI NIH HHS/United States
- U01 HL066491/HL/NHLBI NIH HHS/United States
- 5U01HL068973/HL/NHLBI NIH HHS/United States
- 5U01HL064257/HL/NHLBI NIH HHS/United States
- R37AG018915/AG/NIA NIH HHS/United States
- U01 HL064257/HL/NHLBI NIH HHS/United States
- 5U01HL064264/HL/NHLBI NIH HHS/United States
- R01 NR011873/NR/NINR NIH HHS/United States
- U01 HL066482/HL/NHLBI NIH HHS/United States
- 5U01HL063747/HL/NHLBI NIH HHS/United States
- 5U01HL066491/HL/NHLBI NIH HHS/United States
- U01 HL066494/HL/NHLBI NIH HHS/United States
- 5U01HL066501/HL/NHLBI NIH HHS/United States
- U01 HL066461/HL/NHLBI NIH HHS/United States
- U01 HL068973/HL/NHLBI NIH HHS/United States
- 5U01HL068980/HL/NHLBI NIH HHS/United States
- U01 HL063747/HL/NHLBI NIH HHS/United States
- 5U01HL066494/HL/NHLBI NIH HHS/United States
- U01 HL066501/HL/NHLBI NIH HHS/United States
- 5R01NR011873-02/NR/NINR NIH HHS/United States
- 5U01HL066497/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous