ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling
- PMID: 22763012
- PMCID: PMC3467332
- DOI: 10.1016/j.ceb.2012.06.003
ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling
Abstract
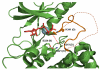
Integrin-linked kinase (ILK) is a widely expressed and evolutionally conserved component of cell-extracellular matrix (ECM) adhesions. Although initially named as a kinase, ILK contains an unusual pseudoactive site that is incapable of catalyzing phosphorylation. Instead, ILK acts as a central component of a heterotrimer (the PINCH-ILK-parvin complex) at ECM adhesions mediating interactions with a large number of proteins via multiple sites including its pseudoactive site. Through higher level protein-protein interactions, this scaffold links integrins to the actin cytoskeleton and catalytic proteins and thereby regulates focal adhesion assembly, cytoskeleton organization and signaling. This review summarizes recent advances in our understanding of the structure and functions of the PINCH-ILK-parvin complex, and discusses emerging new features of the molecular mechanisms by which it regulates diverse cellular, physiological and pathological processes.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

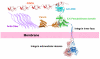
References
-
- Legate KR, Fässler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122(Pt 2):187–98. - PubMed
-
- Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature. 1996;379:91–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

