Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice
- PMID: 22763451
- PMCID: PMC3615424
- DOI: 10.1038/nature11310
Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice
Abstract
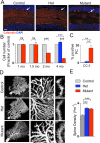
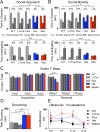
Autism spectrum disorders (ASDs) are highly prevalent neurodevelopmental disorders, but the underlying pathogenesis remains poorly understood. Recent studies have implicated the cerebellum in these disorders, with post-mortem studies in ASD patients showing cerebellar Purkinje cell (PC) loss, and isolated cerebellar injury has been associated with a higher incidence of ASDs. However, the extent of cerebellar contribution to the pathogenesis of ASDs remains unclear. Tuberous sclerosis complex (TSC) is a genetic disorder with high rates of comorbid ASDs that result from mutation of either TSC1 or TSC2, whose protein products dimerize and negatively regulate mammalian target of rapamycin (mTOR) signalling. TSC is an intriguing model to investigate the cerebellar contribution to the underlying pathogenesis of ASDs, as recent studies in TSC patients demonstrate cerebellar pathology and correlate cerebellar pathology with increased ASD symptomatology. Functional imaging also shows that TSC patients with ASDs display hypermetabolism in deep cerebellar structures, compared to TSC patients without ASDs. However, the roles of Tsc1 and the sequelae of Tsc1 dysfunction in the cerebellum have not been investigated so far. Here we show that both heterozygous and homozygous loss of Tsc1 in mouse cerebellar PCs results in autistic-like behaviours, including abnormal social interaction, repetitive behaviour and vocalizations, in addition to decreased PC excitability. Treatment of mutant mice with the mTOR inhibitor, rapamycin, prevented the pathological and behavioural deficits. These findings demonstrate new roles for Tsc1 in PC function and define a molecular basis for a cerebellar contribution to cognitive disorders such as autism.
Figures


Comment in
-
Neurodevelopmental disorders: TSCerebellar autism in mice.Nat Rev Neurosci. 2012 Jul 18;13(8):518. doi: 10.1038/nrn3305. Nat Rev Neurosci. 2012. PMID: 22805910 No abstract available.
-
Defining the role of cerebellar Purkinje cells in autism spectrum disorders.Cerebellum. 2013 Dec;12(6):950-5. doi: 10.1007/s12311-013-0490-y. Cerebellum. 2013. PMID: 23703312 Free PMC article.
References
-
- Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58:1–20. - PubMed
-
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23:183–187. - PubMed
-
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. - PubMed
-
- Limperopoulos C, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–593. - PubMed
-
- Jeste SS, Sahin M, Bolton P, Ploubidis GB, Humphrey A. Characterization of autism in young children with tuberous sclerosis complex. J Child Neurol. 2008;23:520–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

