Human and animal dirofilariasis: the emergence of a zoonotic mosaic
- PMID: 22763636
- PMCID: PMC3416488
- DOI: 10.1128/CMR.00012-12
Human and animal dirofilariasis: the emergence of a zoonotic mosaic
Abstract
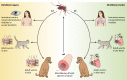
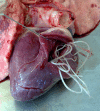
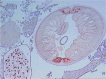
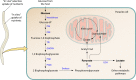
Dirofilariasis represents a zoonotic mosaic, which includes two main filarial species (Dirofilaria immitis and D. repens) that have adapted to canine, feline, and human hosts with distinct biological and clinical implications. At the same time, both D. immitis and D. repens are themselves hosts to symbiotic bacteria of the genus Wolbachia, the study of which has resulted in a profound shift in the understanding of filarial biology, the mechanisms of the pathologies that they produce in their hosts, and issues related to dirofilariasis treatment. Moreover, because dirofilariasis is a vector-borne transmitted disease, their distribution and infection rates have undergone significant modifications influenced by global climate change. Despite advances in our knowledge of D. immitis and D. repens and the pathologies that they inflict on different hosts, there are still many unknown aspects of dirofilariasis. This review is focused on human and animal dirofilariasis, including the basic morphology, biology, protein composition, and metabolism of Dirofilaria species; the climate and human behavioral factors that influence distribution dynamics; the disease pathology; the host-parasite relationship; the mechanisms involved in parasite survival; the immune response and pathogenesis; and the clinical management of human and animal infections.
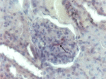
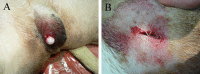
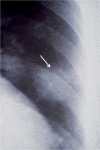
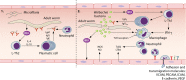
Figures











References
-
- Abraham D, Grieve RD, Oaks JA. 1990. Dirofilaria immitis: moulting process of third-stage larvae. Exp. Parasitol. 70:314–322 - PubMed
-
- Adhami J, Reiter P. 1998. Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J. Am. Mosq. Control Assoc. 14:340–343 - PubMed
-
- Ahid SM, Lourenço-De-Oliveira R. 1999. Mosquitoes potential vectors of canine heartworm in the Northeast Region from Brazil. Rev. Saude Publica 33:560–565 - PubMed
-
- Aiello A, Aiello P, Aiello F. 2005. A case of palpebral dirofilariasis. Eur. J. Ophthalmol. 15:407–408 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

