Hyperoxia resensitizes chemoresistant human glioblastoma cells to temozolomide
- PMID: 22763762
- PMCID: PMC3434886
- DOI: 10.1007/s11060-012-0923-3
Hyperoxia resensitizes chemoresistant human glioblastoma cells to temozolomide
Abstract
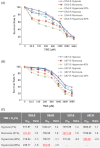
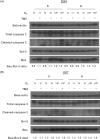
Temozolomide (TMZ) is standard chemotherapy for glioblastoma multiforme (GBM). Intratumoral hypoxia is common in GBM and may be associated with the development of TMZ resistance. Oxygen therapy has previously been reported to potentiate the effect of chemotherapy in cancer. In this study, we investigated whether hyperoxia can enhance the TMZ-induced cytotoxicity of human GBM cells, and whether and how it would resensitize TMZ-resistant GBM cells to TMZ. TMZ-sensitive human GBM cells (D54-S and U87-S) were treated with TMZ to develop isogenic subclones of TMZ-resistant cells (D54-R and U87-R). All cell lines were then exposed to different oxygen levels (1, 21, 40, or 80 %), with or without concomitant TMZ treatment, before assessment of cell cytotoxicity and morphology. Cell death and survival pathways elicited by TMZ and/or hyperoxia were elucidated by western blotting. Our results showed that TMZ sensitivity of both chemo-sensitive and resistant cells was enhanced significantly under hyperoxia. At the cell line-specific optimum oxygen concentration (D54-R, 80 %; U87-R, 40 %), resistant cells had the same response to TMZ as the parent chemosensitive cells under normoxia via the caspase-dependent pathway. Both TMZ and hyperoxia were associated with increased phosphorylation of ERK p44/42 MAPK (Erk1/2), but to a lesser extent in D54-R cells, suggesting that Erk1/2 activity may be involved in regulation of hyperoxia and TMZ-mediated cell death. Overall, hyperoxia enhanced TMZ toxicity in GBM cells by induction of apoptosis, possibly via MAPK-related pathways. Induced hyperoxia is a potentially promising approach for treatment of TMZ-resistant GBM.
Figures



References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Chakravarti A, Palanichamy K. Overcoming therapeutic resistance in malignant gliomas: current practices and future directions. Cancer Treat Res. 2008;139:173–189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

