Progressive alopecia reveals decreasing stem cell activation probability during aging of mice with epidermal deletion of DNA methyltransferase 1
- PMID: 22763785
- PMCID: PMC3465630
- DOI: 10.1038/jid.2012.206
Progressive alopecia reveals decreasing stem cell activation probability during aging of mice with epidermal deletion of DNA methyltransferase 1
Erratum in
- J Invest Dermatol. 2013 Mar;133(3):859. Yu, Juehua [added]
Abstract
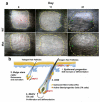
To examine the roles of epigenetic modulation on hair follicle regeneration, we generated mice with a K14-Cre-mediated loss of DNA methyltransferase 1 (DNMT1). The mutant shows an uneven epidermal thickness and alterations in hair follicle size. When formed, hair follicle architecture and differentiation appear normal. Hair subtypes exist but hair fibers are shorter and thinner. Hair numbers appear normal at birth but gradually decrease to <50% of control in 1-year-old mice. Sections of old mutant skin show follicles in prolonged telogen with hyperplastic sebaceous glands. Anagen follicles in mutants exhibit decreased proliferation and increased apoptosis in matrix transient-amplifying cells. Although K15-positive stem cells in the mutant bulge are comparable in number to the control, their ability to proliferate and become activated to form a hair germ is reduced. As mice age, residual DNMT activity declines further, and the probability of successful anagen reentry decreases, leading to progressive alopecia. Paradoxically, there is increased proliferation in the epidermis, which also shows aberrant differentiation. These results highlight the importance of DNA methylation in maintaining stem cell homeostasis during the development and regeneration of ectodermal organs.
Figures
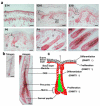
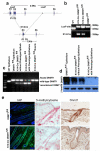
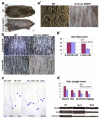
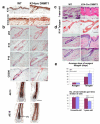

References
-
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–68. - PubMed
-
- Botchkarev VA, Paus R. Molecular biology of hair morphogenesis: development and cycling. J Exp Zool B Mol Dev Evol. 2003;298:164–80. - PubMed
-
- Casillas MA, Jr, Lopatina N, Andrews LG, Tollefsbol TO. Transcriptional control of the DNA methyltransferases is altered in aging and neoplastically-transformed human fibroblasts. Mol Cell Biochem. 2003;252:33–43. - PubMed
-
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

