Spectroscopic study of porphyrin-caffeine interactions
- PMID: 22763925
- PMCID: PMC3473192
- DOI: 10.1007/s10895-012-1090-9
Spectroscopic study of porphyrin-caffeine interactions
Abstract
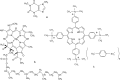
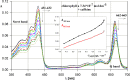
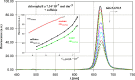
The association between water-soluble porphyrins: 4,4',4″,4'''-(21 H,23 H-porphine-5,10,15,20-tetrayl)tetrakis-(benzoic acid) (H(2)TCPP), 5,10,15,20-tetrakis(4-sulfonatophenyl)-21 H,23 H-porphine (H(2)TPPS(4)), 5,10,15,20-tetrakis[4-(trimethylammonio)phenyl]-21 H,23 H-porphine tetra-p-tosylate (H(2)TTMePP), 5,10,15,20-tetrakis(1-methyl-4-pyridyl)-21 H,23 H-porphine tetra-p-tosylate (H(2)TMePyP), the Cu(II) complexes of H(2)TTMePP and H(2)TMePyP, as well as chlorophyll a with caffeine (1,3,7-trimethylxanthine) has been studied analysing their absorption and emission spectra in aqueous (or acetone in case of chlorophyll a) solution. During the titration by caffeine the porphyrins absorption spectra undergo the evolution - the bathochromic effect can be observed as well as the hypochromicity of the Soret maximum. The association constants were calculated using curve-fitting procedure (K(AC) of the order of magnitude of 10(3) mol(-1)). Whereas the emission spectra point at the presence of the fluorescence quenching effect testifying for the partial inactivation of the porphyrin molecule. The fluorescence quenching constants were calculated from Stern-Volmer plots. The results obtained show that caffeine can interact with water-soluble porphyrins and through formation of stacking complexes is able to quench their ability to emission.
Figures



References
-
- Piosik J, Gwizdek-Wisniewska A, Ulanowska K, Ochocinski J, Czyz A, Wegrzyn G. Methylxanthines (caffeine, pentoxifylline and theophylline) decrease the mutagenic effect of daunomycin, doxorubicin and mitoxantrone. Acta Biochim Pol. 2005;52:923–926. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

