Identification of a novel developmental mechanism in the generation of mesothelia
- PMID: 22764055
- PMCID: PMC3403102
- DOI: 10.1242/dev.082396
Identification of a novel developmental mechanism in the generation of mesothelia
Abstract
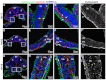
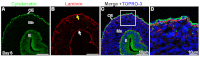
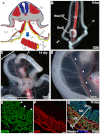
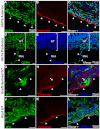
Mesothelium is the surface layer of all coelomic organs and is crucial for the generation of their vasculature. Still, our understanding of the genesis of this essential cell type is restricted to the heart where a localized exogenous population of cells, the proepicardium, migrates to and envelops the myocardium supplying mesothelial, vascular and stromal cell lineages. Currently it is not known whether this pattern of development is specific to the heart or applies broadly to other coelomic organs. Using two independent long-term lineage-tracing studies, we demonstrate that mesothelial progenitors of the intestine are intrinsic to the gut tube anlage. Furthermore, a novel chick-quail chimera model of gut morphogenesis reveals these mesothelial progenitors are broadly distributed throughout the gut primordium and are not derived from a localized and exogenous proepicardium-like source of cells. These data demonstrate an intrinsic origin of mesothelial cells to a coelomic organ and provide a novel mechanism for the generation of mesothelial cells.
Figures




References
-
- Acencio M. M., Vargas F. S., Marchi E., Carnevale G. G., Teixeira L. R., Antonangelo L., Broaddus V. C. (2007). Pleural mesothelial cells mediate inflammatory and profibrotic responses in talc-induced pleurodesis. Lung 185, 343–348 - PubMed
-
- Aroeira L. S., Aguilera A., Selgas R., Ramírez-Huesca M., Pérez-Lozano M. L., Cirugeda A., Bajo M. A., del Peso G., Sánchez-Tomero J. A., Jiménez-Heffernan J. A., et al. (2005). Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: role of vascular endothelial growth factor. Am. J. Kidney Dis. 46, 938–948 - PubMed
-
- Braun N., Alscher D. M., Fritz P., Edenhofer I., Kimmel M., Gaspert A., Reimold F., Bode-Lesniewska B., Ziegler U., Biegger D., et al. (2011). Podoplanin-positive cells are a hallmark of encapsulating peritoneal sclerosis. Nephrol. Dial. Transplant. 26, 1033–1041 - PubMed
-
- Chegini N. (2008). TGF-beta system: the principal profibrotic mediator of peritoneal adhesion formation. Semin. Reprod. Med. 26, 298–312 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK58404/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- R01 DK083234/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- R01 DK83234/DK/NIDDK NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

