Low molecular weight, non-peptidic agonists of TrkA receptor with NGF-mimetic activity
- PMID: 22764098
- PMCID: PMC3406579
- DOI: 10.1038/cddis.2012.80
Low molecular weight, non-peptidic agonists of TrkA receptor with NGF-mimetic activity
Erratum in
-
Low molecular weight, non-peptidic agonists of TrkA receptor with NGF-mimetic activity.Cell Death Dis. 2012 Sep 6;3(9):e389. doi: 10.1038/cddis.2012.129. Cell Death Dis. 2012. PMID: 22951986 Free PMC article.
Abstract
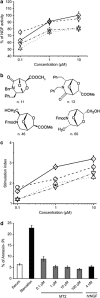
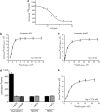
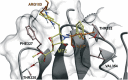
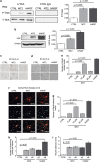
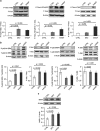
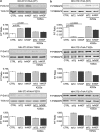
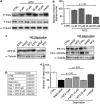
Exploitation of the biologic activity of neurotrophins is desirable for medical purposes, but their protein nature intrinsically bears adverse pharmacokinetic properties. Here, we report synthesis and biologic characterization of a novel class of low molecular weight, non-peptidic compounds with NGF (nerve growth factor)-mimetic properties. MT2, a representative compound, bound to Trk (tropomyosin kinase receptor)A chain on NGF-sensitive cells, as well as in cell-free assays, at nanomolar concentrations and induced TrkA autophosphorylation and receptor-mediated internalization. MT2 binding involved at least two amino-acid residues within TrkA molecule. Like NGF, MT2 increased phosphorylation of extracellular signal-regulated kinase1/2 and Akt proteins and production of MKP-1 phosphatase (dual specificity phosphatase 1), modulated p38 mitogen-activated protein kinase activation, sustained survival of serum-starved PC12 or RDG cells, and promoted their differentiation. However, the intensity of such responses was heterogenous, as the ability of maintaining survival was equally possessed by NGF and MT2, whereas the induction of differentiation was expressed at definitely lower levels by the mimetic. Analysis of TrkA autophosphorylation patterns induced by MT2 revealed a strong tyrosine (Tyr)490 and a limited Tyr785 and Tyr674/675 activation, findings coherent with the observed functional divarication. Consistently, in an NGF-deprived rat hippocampal neuronal model of Alzheimer Disease, MT2 could correct the biochemical abnormalities and sustain cell survival. Thus, NGF mimetics may reveal interesting investigational tools in neurobiology, as well as promising drug candidates.
Figures
References
-
- Barker PA. High affinity not in the vicinity. Neuron. 2007;53:1–4. - PubMed
-
- Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53:25–38. - PubMed
-
- Wiesmann C, Ultsch MH, Bass SH, de Vos AM. Crystal structure of nerve growth factor in complex with the ligand-binding domain of the TrkA receptor. Nature. 1999;401:184–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

