ALS-associated ataxin 2 polyQ expansions enhance stress-induced caspase 3 activation and increase TDP-43 pathological modifications
- PMID: 22764223
- PMCID: PMC3418890
- DOI: 10.1523/JNEUROSCI.0996-12.2012
ALS-associated ataxin 2 polyQ expansions enhance stress-induced caspase 3 activation and increase TDP-43 pathological modifications
Abstract
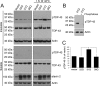
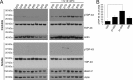
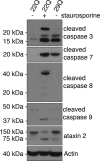
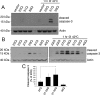
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease caused by the loss of motor neurons. The degenerating motor neurons of ALS patients are characterized by the accumulation of cytoplasmic inclusions containing phosphorylated and truncated forms of the RNA-binding protein TDP-43. Ataxin 2 intermediate-length polyglutamine (polyQ) expansions were recently identified as a risk factor for ALS; however, the mechanism by which they contribute to disease is unknown. Here, we show that intermediate-length ataxin 2 polyQ expansions enhance stress-induced TDP-43 C-terminal cleavage and phosphorylation in human cells. We also connect intermediate-length ataxin 2 polyQ expansions to the stress-dependent activation of multiple caspases, including caspase 3. Caspase activation is upstream of TDP-43 cleavage and phosphorylation since caspase inhibitors block these pathological modifications. Analysis of the accumulation of activated caspase 3 in motor neurons revealed a striking association with ALS cases harboring ataxin 2 polyQ expansions. These findings indicate that activated caspase 3 defines a new pathological feature of ALS with intermediate-length ataxin 2 polyQ expansions. These results provide mechanistic insight into how ataxin 2 intermediate-length polyQ expansions could contribute to ALS--by enhancing stress-induced TDP-43 pathological modifications via caspase activation. Because longer ataxin 2 polyQ expansions are associated with a different disease, spinocerebellar ataxia 2, these findings help explain how different polyQ expansions in the same protein can have distinct cellular consequences, ultimately resulting in different clinical features. Finally, since caspase inhibitors are effective at reducing TDP-43 pathological modifications, this pathway could be pursued as a therapeutic target in ALS.
Figures

Similar articles
-
Distinct TDP-43 pathology in ALS patients with ataxin 2 intermediate-length polyQ expansions.Acta Neuropathol. 2012 Aug;124(2):221-30. doi: 10.1007/s00401-012-0985-5. Epub 2012 Apr 21. Acta Neuropathol. 2012. PMID: 22526021 Free PMC article.
-
Evaluating the prevalence of polyglutamine repeat expansions in amyotrophic lateral sclerosis.Neurology. 2011 Jun 14;76(24):2062-5. doi: 10.1212/WNL.0b013e31821f4447. Epub 2011 May 11. Neurology. 2011. PMID: 21562248 Free PMC article.
-
Ataxin-2 intermediate-length polyglutamine expansions in European ALS patients.Hum Mol Genet. 2011 May 1;20(9):1697-700. doi: 10.1093/hmg/ddr045. Epub 2011 Feb 3. Hum Mol Genet. 2011. PMID: 21292779 Free PMC article.
-
Model organisms reveal insight into human neurodegenerative disease: ataxin-2 intermediate-length polyglutamine expansions are a risk factor for ALS.J Mol Neurosci. 2011 Nov;45(3):676-83. doi: 10.1007/s12031-011-9548-9. Epub 2011 Jun 10. J Mol Neurosci. 2011. PMID: 21660502 Free PMC article. Review.
-
Taking a risk: a therapeutic focus on ataxin-2 in amyotrophic lateral sclerosis?Trends Mol Med. 2014 Jan;20(1):25-35. doi: 10.1016/j.molmed.2013.09.001. Epub 2013 Oct 16. Trends Mol Med. 2014. PMID: 24140266 Review.
Cited by
-
TAR DNA-Binding Protein 43 is Cleaved by the Protease 3C of Enterovirus A71.Virol Sin. 2021 Feb;36(1):95-103. doi: 10.1007/s12250-020-00262-x. Epub 2020 Jul 21. Virol Sin. 2021. PMID: 32696397 Free PMC article.
-
The changing scene of amyotrophic lateral sclerosis.Nat Rev Neurosci. 2013 Apr;14(4):248-64. doi: 10.1038/nrn3430. Epub 2013 Mar 6. Nat Rev Neurosci. 2013. PMID: 23463272 Review.
-
The roles of intrinsic disorder-based liquid-liquid phase transitions in the "Dr. Jekyll-Mr. Hyde" behavior of proteins involved in amyotrophic lateral sclerosis and frontotemporal lobar degeneration.Autophagy. 2017;13(12):2115-2162. doi: 10.1080/15548627.2017.1384889. Epub 2017 Dec 17. Autophagy. 2017. PMID: 28980860 Free PMC article. Review.
-
ALS-linked mutations enlarge TDP-43-enriched neuronal RNA granules in the dendritic arbor.J Neurosci. 2014 Mar 19;34(12):4167-74. doi: 10.1523/JNEUROSCI.2350-13.2014. J Neurosci. 2014. PMID: 24647938 Free PMC article.
-
Amyotrophic lateral sclerosis and spinocerebellar ataxia type 2 in a family with full CAG repeat expansions of ATXN2.JAMA Neurol. 2013 Oct;70(10):1302-4. doi: 10.1001/jamaneurol.2013.443. JAMA Neurol. 2013. PMID: 23959108 Free PMC article.
References
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. - PubMed
-
- Brady OA, Meng P, Zheng Y, Mao Y, Hu F. Regulation of TDP-43 aggregation by phosphorylation and p62/SQSTM1. J Neurochem. 2011;116:248–259. - PubMed
-
- Chen Y, Huang R, Yang Y, Chen K, Song W, Pan P, Li J, Shang HF. Ataxin-2 intermediate-length polyglutamine: a possible risk factor for Chinese patients with amyotrophic lateral sclerosis. Neurobiol Aging. 2011;32:1925.e1–e5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
