Spectraplakins promote microtubule-mediated axonal growth by functioning as structural microtubule-associated proteins and EB1-dependent +TIPs (tip interacting proteins)
- PMID: 22764224
- PMCID: PMC3666083
- DOI: 10.1523/JNEUROSCI.0416-12.2012
Spectraplakins promote microtubule-mediated axonal growth by functioning as structural microtubule-associated proteins and EB1-dependent +TIPs (tip interacting proteins)
Abstract
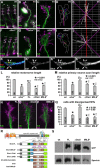
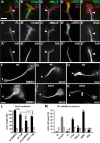
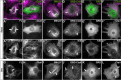
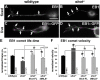
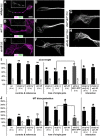
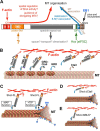
The correct outgrowth of axons is essential for the development and regeneration of nervous systems. Axon growth is primarily driven by microtubules. Key regulators of microtubules in this context are the spectraplakins, a family of evolutionarily conserved actin-microtubule linkers. Loss of function of the mouse spectraplakin ACF7 or of its close Drosophila homolog Short stop/Shot similarly cause severe axon shortening and microtubule disorganization. How spectraplakins perform these functions is not known. Here we show that axonal growth-promoting roles of Shot require interaction with EB1 (End binding protein) at polymerizing plus ends of microtubules. We show that binding of Shot to EB1 requires SxIP motifs in Shot's C-terminal tail (Ctail), mutations of these motifs abolish Shot functions in axonal growth, loss of EB1 function phenocopies Shot loss, and genetic interaction studies reveal strong functional links between Shot and EB1 in axonal growth and microtubule organization. In addition, we report that Shot localizes along microtubule shafts and stabilizes them against pharmacologically induced depolymerization. This function is EB1-independent but requires net positive charges within Ctail which essentially contribute to the microtubule shaft association of Shot. Therefore, spectraplakins are true members of two important classes of neuronal microtubule regulating proteins: +TIPs (tip interacting proteins; plus end regulators) and structural MAPs (microtubule-associated proteins). From our data we deduce a model that relates the different features of the spectraplakin C terminus to the two functions of Shot during axonal growth.
Figures




References
-
- Ahmad FJ, He Y, Myers KA, Hasaka TP, Francis F, Black MM, Baas PW. Effects of dynactin disruption and dynein depletion on axonal microtubules. Traffic. 2006;7:524–537. - PubMed
-
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- 092403/Z/10/Z/WT_/Wellcome Trust/United Kingdom
- 078593/Z/05/Z/WT_/Wellcome Trust/United Kingdom
- BB/C515998/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 077748/WT_/Wellcome Trust/United Kingdom
- 087820/Z/08/Z/WT_/Wellcome Trust/United Kingdom
- 077748/Z/05/Z/WT_/Wellcome Trust/United Kingdom
- 074343/Z/04/B/WT_/Wellcome Trust/United Kingdom
- BB/I002448/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 092403/Z/10/Z/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
