Modulation of circuit feedback specifies motor circuit output
- PMID: 22764227
- PMCID: PMC3398468
- DOI: 10.1523/JNEUROSCI.1461-12.2012
Modulation of circuit feedback specifies motor circuit output
Abstract
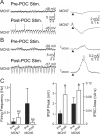
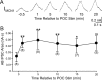
Bidirectional communication (i.e., feedforward and feedback pathways) between functional levels is common in neural systems, but in most systems little is known regarding the function and modifiability of the feedback pathway. We are exploring this issue in the crab (Cancer borealis) stomatogastric nervous system by examining bidirectional communication between projection neurons and their target central pattern generator (CPG) circuit neurons. Specifically, we addressed the question of whether the peptidergic post-oesophageal commissure (POC) neurons trigger a specific gastric mill (chewing) motor pattern in the stomatogastric ganglion solely by activating projection neurons, or by additionally altering the strength of CPG feedback to these projection neurons. The POC-triggered gastric mill rhythm is shaped by feedback inhibition onto projection neurons from a CPG neuron. Here, we establish that POC stimulation triggers a long-lasting enhancement of feedback-mediated IPSC/Ps in the projection neurons, which persists for the same duration as POC-gastric mill rhythms. This strengthened CPG feedback appears to result from presynaptic modulation, because it also occurs in other projection neurons whose activity does not change after POC stimulation. To determine the function of this strengthened feedback synapse, we compared the influence of dynamic-clamp-injected feedback IPSPs of pre- and post-POC amplitude into a pivotal projection neuron after POC stimulation. Only the post-POC amplitude IPSPs elicited the POC-triggered activity pattern in this projection neuron and enabled full expression of the POC-gastric mill rhythm. Thus, the strength of CPG feedback to projection neurons is modifiable and can be instrumental to motor pattern selection.
Figures







Similar articles
-
State-dependent presynaptic inhibition regulates central pattern generator feedback to descending inputs.J Neurosci. 2008 Sep 17;28(38):9564-74. doi: 10.1523/JNEUROSCI.3011-08.2008. J Neurosci. 2008. PMID: 18799688 Free PMC article.
-
State-dependent sensorimotor gating in a rhythmic motor system.J Neurophysiol. 2017 Nov 1;118(5):2806-2818. doi: 10.1152/jn.00420.2017. Epub 2017 Aug 16. J Neurophysiol. 2017. PMID: 28814634 Free PMC article.
-
Circuit feedback increases activity level of a circuit input through interactions with intrinsic properties.J Neurophysiol. 2017 Aug 1;118(2):949-963. doi: 10.1152/jn.00772.2016. Epub 2017 May 3. J Neurophysiol. 2017. PMID: 28469000 Free PMC article.
-
Frequency control of a slow oscillatory network by a fast rhythmic input: pyloric to gastric mill interactions in the crab stomatogastric nervous system.Ann N Y Acad Sci. 1998 Nov 16;860:226-38. doi: 10.1111/j.1749-6632.1998.tb09052.x. Ann N Y Acad Sci. 1998. PMID: 9928315 Review.
-
Neural circuit flexibility in a small sensorimotor system.Curr Opin Neurobiol. 2011 Aug;21(4):544-52. doi: 10.1016/j.conb.2011.05.019. Epub 2011 Jun 30. Curr Opin Neurobiol. 2011. PMID: 21689926 Free PMC article. Review.
Cited by
-
Energy-adaptive resistive switching with controllable thresholds in insulator-metal transition.RSC Adv. 2022 Dec 12;12(55):35579-35586. doi: 10.1039/d2ra06866d. eCollection 2022 Dec 12. RSC Adv. 2022. PMID: 36540398 Free PMC article.
-
A modeling exploration of how synaptic feedback to descending projection neurons shapes the activity of an oscillatory network.SIAM J Appl Dyn Syst. 2014 Aug 12;13(3):1239-1269. doi: 10.1137/130943881. SIAM J Appl Dyn Syst. 2014. PMID: 25419188 Free PMC article.
-
QUANTITATIVE REEVALUATION OF THE EFFECTS OF SHORT- AND LONG-TERM REMOVAL OF DESCENDING MODULATORY INPUTS ON THE PYLORIC RHYTHM OF THE CRAB, CANCER BOREALIS.eNeuro. 2015 Jan;2(1):ENEURO.0058-14.2015. doi: 10.1523/ENEURO.0058-14.2015. eNeuro. 2015. PMID: 25914899 Free PMC article.
-
Multiple intrinsic membrane properties are modulated in a switch from single- to dual-network activity.J Neurophysiol. 2022 Nov 1;128(5):1181-1198. doi: 10.1152/jn.00337.2022. Epub 2022 Oct 5. J Neurophysiol. 2022. PMID: 36197020 Free PMC article.
-
Neural circuit regulation by identified modulatory projection neurons.Front Neurosci. 2023 Mar 17;17:1154769. doi: 10.3389/fnins.2023.1154769. eCollection 2023. Front Neurosci. 2023. PMID: 37008233 Free PMC article. Review.
References
-
- Antri M, Auclair F, Albrecht J, Djeudjang N, Dubuc R. Serotoninergic modulation of sensory transmission to brainstem reticulospinal cells. Eur J Neurosci. 2008;28:655–667. - PubMed
-
- Arshavsky YI, Orlovsky GN, Perret C. Activity of rubrospinal neurons during locomotion and scratching in the cat. Behav Brain Res. 1988;28:193–199. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
