Using motor behavior during an early critical period to restore skilled limb movement after damage to the corticospinal system during development
- PMID: 22764234
- PMCID: PMC3422625
- DOI: 10.1523/JNEUROSCI.1198-12.2012
Using motor behavior during an early critical period to restore skilled limb movement after damage to the corticospinal system during development
Abstract
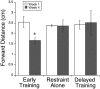
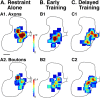
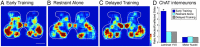
This study investigated the requirements for restoring motor function after corticospinal (CS) system damage during early postnatal development. Activity-dependent competition between the CS tracts (CSTs) of the two hemispheres is imperative for normal development. Blocking primary motor cortex (M1) activity unilaterally during a critical period [postnatal week 5 (PW5) to PW7] produces permanent contralateral motor skill impairments, loss of M1 motor map, aberrant CS terminations, and decreases in CST presynaptic sites and spinal cholinergic interneuron numbers. To repair these motor systems impairments and restore function, we manipulated motor experience in three groups of cats after this CST injury produced by inactivation. One group wore a jacket restraining the limb ipsilateral to inactivation, forcing use of the contralateral, impaired limb, for the month after M1 inactivation (PW8-PW13; "restraint alone"). A second group wore the restraint during PW8-PW13 and was also trained for 1 h/d in a reaching task with the contralateral forelimb ("early training"). To test the efficacy of intervention during adolescence, a third group wore the restraint and received reach training during PW20-PW24 ("delayed training"). Early training restored CST connections and the M1 motor map, increased cholinergic spinal interneurons numbers on the contralateral, relative to ipsilateral, side, and abrogated limb control impairments. Delayed training restored CST connectivity and the M1 motor map but not contralateral spinal cholinergic cell counts or motor performance. Restraint alone only restored CST connectivity. Our findings stress the need to reestablish the integrated functions of the CS system at multiple hierarchical levels in restoring skilled motor function after developmental injury.
Figures






References
-
- Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984;229:329–346. - PubMed
-
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. - PubMed
-
- Chakrabarty S, Martin JH. Postnatal development of the motor representation in primary motor cortex. J Neurophysiol. 2000;84:2582–2594. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
