TRPC5 channel is the mediator of neurotrophin-3 in regulating dendritic growth via CaMKIIα in rat hippocampal neurons
- PMID: 22764246
- PMCID: PMC6622237
- DOI: 10.1523/JNEUROSCI.6363-11.2012
TRPC5 channel is the mediator of neurotrophin-3 in regulating dendritic growth via CaMKIIα in rat hippocampal neurons
Abstract
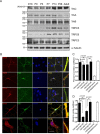
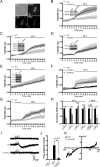
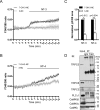
Neurotrophin-3 (NT-3) plays numerous important roles in the CNS and the elevation of intracellular Ca(2+) ([Ca(2+)](i)) is critical for these functions of NT-3. However, the mechanism by which NT-3 induces [Ca(2+)](i) elevation remains largely unknown. Here, we found that transient receptor potential canonical (TRPC) 5 protein and TrkC, the NT-3 receptor, exhibited a similar temporal expression in rat hippocampus and cellular colocalization in hippocampal neurons. Stimulation of the neurons by NT-3 induced a nonselective cation conductance and PLCγ-dependent [Ca(2+)](i) elevation, which were both blocked when TRPC5, but not TRPC6 channels, were inhibited. Moreover, the Ca(2+) influx through TRPC5 induced by NT-3 inhibited the neuronal dendritic growth through activation of calmodulin-dependent kinase (CaMK) IIα. In contrast, the Ca(2+) influx through TRPC6 induced by NT-4 promoted the dendritic growth. Thus, TRPC5 acts as a novel and specific mediator for NT-3 to regulate dendrite development through CaMKIIα.
Figures





Similar articles
-
TRPC6 channel-mediated neurite outgrowth in PC12 cells and hippocampal neurons involves activation of RAS/MEK/ERK, PI3K, and CAMKIV signaling.J Neurochem. 2013 Nov;127(3):303-13. doi: 10.1111/jnc.12376. Epub 2013 Aug 19. J Neurochem. 2013. PMID: 23875811
-
TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway.J Cell Sci. 2008 Jul 15;121(Pt 14):2301-7. doi: 10.1242/jcs.026906. Epub 2008 Jun 17. J Cell Sci. 2008. PMID: 18559891
-
A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain.Genes Dev. 2011 Dec 15;25(24):2659-73. doi: 10.1101/gad.174060.111. Epub 2011 Dec 1. Genes Dev. 2011. PMID: 22135323 Free PMC article.
-
Canonical transient receptor potential 5.Handb Exp Pharmacol. 2007;(179):109-23. doi: 10.1007/978-3-540-34891-7_6. Handb Exp Pharmacol. 2007. PMID: 17217053 Review.
-
TRPC5.Handb Exp Pharmacol. 2014;222:129-56. doi: 10.1007/978-3-642-54215-2_6. Handb Exp Pharmacol. 2014. PMID: 24756705 Review.
Cited by
-
TRPC6 specifically interacts with APP to inhibit its cleavage by γ-secretase and reduce Aβ production.Nat Commun. 2015 Nov 19;6:8876. doi: 10.1038/ncomms9876. Nat Commun. 2015. PMID: 26581893 Free PMC article.
-
Cocaine self-administration in mice with forebrain knock-down of trpc5 ion channels.F1000Res. 2013 Feb 15;2:53. doi: 10.12688/f1000research.2-53.v1. eCollection 2013. F1000Res. 2013. PMID: 24358869 Free PMC article.
-
The role of canonical transient receptor potential channels in seizure and excitotoxicity.Cells. 2014 Apr 9;3(2):288-303. doi: 10.3390/cells3020288. Cells. 2014. PMID: 24722470 Free PMC article.
-
Wireless neuromodulation in vitro and in vivo by intrinsic TRPC-mediated magnetomechanical stimulation.Commun Biol. 2022 Nov 2;5(1):1166. doi: 10.1038/s42003-022-04124-y. Commun Biol. 2022. PMID: 36323817 Free PMC article.
-
Gq-Coupled Muscarinic Receptor Enhancement of KCNQ2/3 Channels and Activation of TRPC Channels in Multimodal Control of Excitability in Dentate Gyrus Granule Cells.J Neurosci. 2019 Feb 27;39(9):1566-1587. doi: 10.1523/JNEUROSCI.1781-18.2018. Epub 2018 Dec 28. J Neurosci. 2019. PMID: 30593498 Free PMC article.
References
-
- Abraham ST, Benscoter HA, Schworer CM, Singer HA. A role for Ca2+/calmodulin-dependent protein kinase II in the mitogen-activated protein kinase signaling cascade of cultured rat aortic vascular smooth muscle cells. Circ Res. 1997;81:575–584. - PubMed
-
- Allan D, Michell RH. A calcium-activated polyphosphoinositide phosphodiesterase in the plasma membrane of human and rabbit erythrocytes. Biochim Biophys Acta. 1978;508:277–286. - PubMed
-
- Andrade R. Cell excitation enhances muscarinic cholinergic responses in rat association cortex. Brain Res. 1991;548:81–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
