Using solid-state NMR to monitor the molecular consequences of Cryptococcus neoformans melanization with different catecholamine precursors
- PMID: 22765382
- PMCID: PMC3448835
- DOI: 10.1021/bi300325m
Using solid-state NMR to monitor the molecular consequences of Cryptococcus neoformans melanization with different catecholamine precursors
Abstract
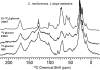
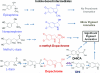
Melanins are a class of natural pigments associated with a wide range of biological functions, including microbial virulence, energy transduction, and protection against solar radiation. Because of their insolubility and structural heterogeneity, solid-state nuclear magnetic resonance (NMR) spectroscopy provides an unprecedented means to define the molecular architecture of these enigmatic pigments. The requirement of obligatory catecholamines for melanization of the pathogenic fungus Cryptococcus neoformans also offers unique opportunities for investigating melanin development. In the current study, pigments produced with L-dopa, methyl-L-dopa, epinephrine, and norepinephrine precursors are compared structurally using (13)C and (1)H magic-angle spinning (MAS) NMR. Striking structural differences were observed for both aromatic and aliphatic molecular constituents of the mature fungal pigment assemblies, thus making it possible to redefine the molecular prerequisites for formation of the aromatic domains of insoluble indole-based biopolymers, to rationalize their distinctive physical characteristics, and to delineate the role of cellular constituents in assembly of the melanized macromolecules with polysaccharides and fatty acyl chain-containing moieties. By achieving an augmented understanding of the mechanisms of C. neoformans melanin biosynthesis and cellular assembly, such studies can guide future drug discovery efforts related to melanin-associated virulence, resistance to tumor therapy, and production of melanin mimetics under cell-free conditions.
Figures


References
-
- Hill HZ. The Function of Melanin or Six Blind People Examine an Elephant. Bioessays. 1992;14:49–56. - PubMed
-
- Bell AA, Wheeler MH. Biosynthesis and Functions of Fungal Melanins. Annual Review of Phytopathology. 1986;24:411–451.
-
- Garcia-Rivera J, Eisenman HC, Nosanchuk JD, Aisen P, Zaragoza O, Moadel T, Dadachova E, Casadevall A. Comparative analysis of Cryptococcus neoformans acid-resistant particles generated from pigmented cells grown in different laccase substrates. Fungal Genetics and Biology. 2005;42:989–998. - PubMed
-
- Langfelder K, Streibel M, Jahn B, Haase G, Brakhage AA. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genetics and Biology. 2003;38:143–158. - PubMed
-
- Tian SY, Garcia-Rivera J, Yan B, Casadevall A, Stark RE. Unlocking the molecular structure of fungal melanin using C 13 biosynthetic labeling and solid-state NMR. Biochemistry. 2003;42:8105–8109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

