Palladium-catalyzed 1,1-difunctionalization of ethylene
- PMID: 22765909
- PMCID: PMC3459322
- DOI: 10.1021/ja304344h
Palladium-catalyzed 1,1-difunctionalization of ethylene
Abstract
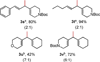
The 1,1-difunctionalization of ethylene, with aryl/vinyl/heteroaryl transmetalating agents and vinyl electrophiles, is reported. The reaction is high-yielding under a low pressure of ethylene, and regioselectivity is generally high for the 1,1-disubstituted product. The process is highlighted by the use of heteroaromatic cross-coupling reagents, which have not been competent reaction partners in previously reported efforts.
Figures

References
-
- McCoy M, Reisch M, Tullo AH, Short PL, Tremblay J-F. Chem. Eng. News. 2006;84(28):59.
-
- Matsubara R, Gutierrez AC, Jamison TF. J. Am. Chem. Soc. 2011;133:19020. - PMC - PubMed
- Atla SB, Kelkar AA, Puranik VG, Bensch W, Chaudhari RV. J. Organomet. Chem. 2009;694:683.
- Kiji J, Okano T, Ooue A. J. Mol. Catal. A: Chem. 1999;147:3.
- Kiji J, Okano T, Hasegawa T. J. Mol. Catal. A: Chem. 1995;97:73.
- Beller M, Fischer H, Kühlein K. Tetrahedron Lett. 1994;35:8773.
- Spencer A. J. Organomet. Chem. 1983;247:117.
- Spencer A. J. Organomet. Chem. 1983;258:101.
- Plevyak JE, Heck RF. J. Org. Chem. 1978;43:2454.
- Heck RF. J. Am. Chem. Soc. 1968;90:5518.
-
- Page JP, RajanBabu TV. J. Am. Chem. Soc. 2012;134:6556. - PMC - PubMed
- Mans DJ, Cox GA, RajanBabu TV. J. Am. Chem. Soc. 2011;133:5776. - PMC - PubMed
- Sharma RK, RajanBabu TV. J. Am. Chem. Soc. 2010;132:3295. - PMC - PubMed
- RajanBabu TV. Chem. Rev. 2003;103:2845. - PubMed
- Francio G, Faraone F, Leitner W. J. Am. Chem. Soc. 2002;124:736. - PubMed
- Nomura N, Jin J, Park H, RajanBabu TV. J. Am. Chem. Soc. 1998;120:459.
-
- Grotevendt AGD, Lummiss JAM, Mastronardi ML, Fogg DE. J. Am. Chem. Soc. 2011;133:15918. - PubMed
- Villar H, Frings M, Bolm C. Chem. Soc. Rev. 2007;36:55. - PubMed
- Giessert AJ, Brazis NJ, Diver ST. Org. Lett. 2003;5:3819. - PubMed
- Tonogaki K, Mori M. Tetrahedron Lett. 2002;43:2235.
- Smulik JA, Diver ST. J. Org. Chem. 2000;65:1788. - PubMed
- Mori M, Sakakibara N, Kinoshita A. J. Org. Chem. 1998;63:6082. - PubMed
- Kinoshita A, Sakakibara N, Mori M. J. Am. Chem. Soc. 1997;119:12388.
-
-
Smidt J, Hafner W, Jira R, Sieber R, Sedlmeier J, Sabel A. Angew. Chem. 1962;74:93. For reviews on Wacker oxidations, see: Cornell CN, Sigman MS. Inorg. Chem. 2007;46:1903. Takacs JM, Jiang X-t. Curr. Org. Chem. 2003;7:369. Tsuji J. Synthesis. 1984;1984:369.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

