Molecular techniques for diagnosis of Clostridium difficile infection: systematic review and meta-analysis
- PMID: 22766084
- PMCID: PMC3538482
- DOI: 10.1016/j.mayocp.2012.02.024
Molecular techniques for diagnosis of Clostridium difficile infection: systematic review and meta-analysis
Abstract
Objective: To assess the usefulness of 2 rapid molecular diagnostic techniques, polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP), in Clostridium difficile infection (CDI).
Methods: We conducted a systematic review and meta-analysis to evaluate the accuracy of PCR and LAMP in diagnosis of CDI, including studies that used toxigenic culture or cytotoxicity assay as reference standard.
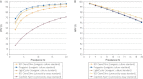
Results: A search of PubMed and CinAHL medical databases yielded 25 PCR studies, including 11,801 samples that met inclusion criteria and 6 heterogeneous studies that evaluated LAMP. With toxigenic culture as a standard, pooled sensitivity was 0.92 (95% confidence interval [CI], 0.91-0.94); specificity, 0.94 (95% CI, 0.94-0.95); and diagnostic odds ratio, 378 (95% CI, 260-547). With cytotoxicity as a standard, pooled sensitivity was 0.87 (95% CI, 0.84-0.90); specificity, 0.97 (95% CI, 0.97-0.98); and diagnostic odds ratio, 370 (95% CI, 226-606).
Conclusion: Polymerase chain reaction is a highly accurate test for identifying CDI. Heterogeneity in LAMP studies did not allow meta-analysis; however, further research into this promising method is warranted.
Copyright © 2012 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Bartlett J.G. Antibiotic-associated diarrhea. Clin Infect Dis. 1992;15(4):573–581. - PubMed
-
- Warny M., Pepin J., Fang A. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491):1079–1084. - PubMed
-
- McDonald L.C., Killgore G.E., Thompson A. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433–2441. - PubMed
-
- Bartlett J.G. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758–764. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

