KLF4 and SOX9 transcription factors antagonize β-catenin and inhibit TCF-activity in cancer cells
- PMID: 22766303
- PMCID: PMC3633466
- DOI: 10.1016/j.bbamcr.2012.06.027
KLF4 and SOX9 transcription factors antagonize β-catenin and inhibit TCF-activity in cancer cells
Abstract
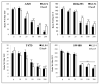
The transcriptional activator β-catenin is a key mediator of the canonical Wnt signaling pathway. β-catenin itself does not bind DNA but functions via interaction with T-cell factor (TCF)/lymphoid-enhancing factor (LEF) transcription factors. Thus, in the case of active Wnt signaling, β-catenin, in cooperation with TCF/LEF proteins family, activates the expression of a wide variety of genes. To date, the list of established β-catenin interacting targets is far from complete. In this study, we aimed to establish the interaction between β-catenin and transcription factors that might affect TCF activity. We took advantage of EMSA, using TCF as a probe, to screen oligonucleotides known to bind specific transcription factors that might dislodge or antagonize β-catenin/TCF binding. We found that Sox9 and KLF4 antagonize β-catenin/TCF binding in HEK293, A549, SW480, and T47D cells. This inhibition of TCF binding was concentration-dependent and correlated to the in vitro TCF-luciferase functional assays. Overexpression of Sox9 and KLF4 transcription factors in cancer cells shows a concentration-dependent reduction of TCF-luciferase as well as the TCF-binding activities. In addition, we demonstrated that both Sox9 and KLF4 interact with β-catenin in an immunoprecipitation assay and reduce its binding to TCF4. Together, these results demonstrate that Sox9 and KLF4 transcription factors antagonize β-catenin/TCF in cancer cells.
Published by Elsevier B.V.
Figures







References
-
- Clevers H. Wnt/β-Catenin Signaling in Development and Disease. Cell. 2006;27:469–480. Review. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

