Pituitary adenylate cyclase-activating polypeptide (PACAP) potently dilates middle meningeal arteries: implications for migraine
- PMID: 22766684
- PMCID: PMC3581331
- DOI: 10.1007/s12031-012-9851-0
Pituitary adenylate cyclase-activating polypeptide (PACAP) potently dilates middle meningeal arteries: implications for migraine
Abstract
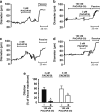
Migraine is a debilitating neurological disorder characterized by mild to severe headache that is often accompanied by aura and other neurological symptoms. Among proposed mechanisms, dilation of the dural vasculature especially the middle meningeal artery (MMA) has been implicated as one component underlying this disorder. Several regulatory peptides from trigeminal sensory and sphenopalatine postganglionic parasympathetic fibers innervating these vessels have been implicated in the process including pituitary adenylate cyclase-activating polypeptide (PACAP). Although PACAP has been well described as a potent dilator in many vascular beds, the effects of PACAP on the dural vasculature are unclear. In the current study, we examined the ability of PACAP to dilate MMAs that were isolated from rats and pressurized ex vivo. PACAP38 potently dilated pressurized MMAs with an EC(50) of 1 pM. The PAC1 receptor antagonist, PACAP(6-38), abolished MMA dilation caused by picomolar concentrations of PACAP. In contrast, cerebellar arteries isolated from the brain surface were ~1,000-fold less sensitive to PACAP than MMAs. Although cerebellar arteries expressed transcripts for all three PACAP receptor subtypes (PAC1, VPAC1, and VPAC2 receptors) by RT-PCR analyses, MMA demonstrated only PAC1 and VPAC2 receptor expression. Further, multiple variants of the PAC1 receptor were identified in the MMA. The expression of PAC1 receptors and the high potency of PACAP to induce MMA vasodilation are consistent with their potential roles in the etiology of migraine.
Figures



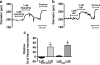
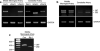
References
-
- Amin FM, Asghar MS, Guo S, et al. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia. 2012;32:140–149. - PubMed
-
- Asghar MS, Hansen AE, Amin FM, et al. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–645. - PubMed
-
- Baun M, Hay-Schmidt A, Edvinsson L, Olesen J, Jansen-Olesen I. Pharmacological characterization and expression of VIP and PACAP receptors in isolated cranial arteries of the rat. Eur J Pharmacol. 2011;670:186–194. - PubMed
-
- Boni LJ, Ploug KB, Olesen J, Jansen-Olesen I, Gupta S. The in vivo effect of VIP, PACAP-38 and PACAP-27 and mRNA expression of their receptors in rat middle meningeal artery. Cephalalgia. 2009;29:837–847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

