Docetaxel combined with irinotecan or 5-fluorouracil in patients with advanced oesophago-gastric cancer: a randomised phase II study
- PMID: 22767144
- PMCID: PMC3405223
- DOI: 10.1038/bjc.2012.286
Docetaxel combined with irinotecan or 5-fluorouracil in patients with advanced oesophago-gastric cancer: a randomised phase II study
Abstract
Background: Docetaxel and irinotecan chemotherapy have shown good efficacy in the treatment of advanced oesophago-gastric cancer. This randomised phase II study evaluated the efficacy and toxicity profile of two non-platinum docetaxel-based doublet regimens in advanced oesophago-gastric cancer.
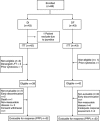
Methods: Chemotherapy-naïve patients with advanced oesophago-gastric cancer were randomised to receive either 3-weekly DI (docetaxel 60 mg m(-2) plus irinotecan 250 mg m(-2) (Day 1)) or 3-weekly DF (docetaxel 85 mg m(-2) (Day 1) followed by 5-fluorouracil 750 mg m(-2) per day as a continuous infusion (Days 1-5)).
Results: A total of 85 patients received DI (n=42) or DF (n=43). The primary endpoint was overall response rate (ORR). The ORR and time to progression (TTP) in the evaluable population (n=65) were 37.5% (DI) vs 33.3% (DF), and 4.2 months vs 4.4 months, respectively. In the intent-to-treat population, the observed ORR, TTP and median overall survival were similar between the two groups. Grade 3-4 neutropenia, febrile neutropenia and diarrhoea were more frequent in the DI arm as compared with the DF arm (83.3% vs 69.8%, 40.5% vs 18.6%, and 42.9% vs 16.3%, respectively).
Conclusion: Both docetaxel-based doublet regimens show comparable efficacy; however, the DF regimen was associated with a better toxicity profile and is an alternative treatment option for patients in whom platinum-based regimens are unsuitable.
© 2012 Cancer Research UK
Figures

References
-
- Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, Majlis A, Assadourian S, Van Cutsem E (2005) Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol 23(24): 5660–5667 - PubMed
-
- Assersohn L, Brown G, Cunningham D, Ward C, Oates J, Waters JS, Hill ME, Norman AR (2004) Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol 15(1): 64–69 - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742): 687–697 - PubMed
-
- Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, Nasu J, Ohtsu A (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 10(11): 1063–1069 - PubMed
-
- Constenla M, Garcia-Arroyo R, Lorenzo I, Carrete N, Campos B, Palacios P (2002) Docetaxel, 5-fluorouracil, and leucovorin as treatment for advanced gastric cancer: results of a phase II study. Gastric Cancer 5(3): 142–147 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

