P2Y receptor subtypes evoke different Ca2+ signals in cultured aortic smooth muscle cells
- PMID: 22767215
- PMCID: PMC3486169
- DOI: 10.1007/s11302-012-9323-6
P2Y receptor subtypes evoke different Ca2+ signals in cultured aortic smooth muscle cells
Abstract
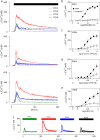
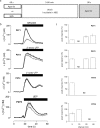
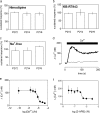
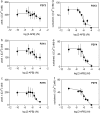
Adenine and uridine nucleotides evoke Ca(2+) signals via four subtypes of P2Y receptor in cultured aortic smooth muscle cells, but the mechanisms underlying the different patterns of these Ca(2+) signals are unresolved. Cytosolic Ca(2+) signals were recorded from single cells and populations of cultured rat aortic smooth muscle cells, loaded with a fluorescent Ca(2+) indicator and stimulated with agonists that allow subtype-selective activation of P2Y1, P2Y2, P2Y4, or P2Y6 receptors. Activation of P2Y1, P2Y2, and P2Y6 receptors caused homologous desensitisation, while activation of P2Y2 receptors also caused heterologous desensitisation of the other subtypes. The Ca(2+) signals evoked by each P2Y receptor subtype required activation of phospholipase C and release of Ca(2+) from intracellular stores via inositol 1,4,5-trisphosphate (IP(3)) receptors, but they were unaffected by inhibition of ryanodine or nicotinic acid adenine dinucleotide phosphate (NAADP) receptors. Sustained Ca(2+) signals were independent of the Na(+)/Ca(2+) exchanger and were probably mediated by store-operated Ca(2+) entry. Analyses of single cells established that most cells express P2Y2 receptors and at least two other P2Y receptor subtypes. We conclude that four P2Y receptor subtypes evoke Ca(2+) signals in cultured aortic smooth muscle cells using the same intracellular (IP(3) receptors) and Ca(2+) entry pathways (store-operated Ca(2+) entry). Different rates of homologous desensitisation and different levels of receptor expression account for the different patterns of Ca(2+) signal evoked by each P2Y receptor subtype.
Figures



Similar articles
-
Ca(2+) signalling by P2Y receptors in cultured rat aortic smooth muscle cells.Br J Pharmacol. 2010 Aug;160(8):1953-62. doi: 10.1111/j.1476-5381.2010.00763.x. Br J Pharmacol. 2010. PMID: 20649593 Free PMC article.
-
ATP inhibits Ins(1,4,5)P3-evoked Ca2+ release in smooth muscle via P2Y1 receptors.J Cell Sci. 2012 Nov 1;125(Pt 21):5151-8. doi: 10.1242/jcs.108498. Epub 2012 Aug 16. J Cell Sci. 2012. PMID: 22899721 Free PMC article.
-
ATP induces contraction of cultured brain capillary pericytes via activation of P2Y-type purinergic receptors.Am J Physiol Heart Circ Physiol. 2021 Feb 1;320(2):H699-H712. doi: 10.1152/ajpheart.00560.2020. Epub 2020 Dec 11. Am J Physiol Heart Circ Physiol. 2021. PMID: 33306443
-
Pharmacology and structure of P2Y receptors.Neuropharmacology. 2016 May;104:50-61. doi: 10.1016/j.neuropharm.2015.10.030. Epub 2015 Oct 28. Neuropharmacology. 2016. PMID: 26519900 Review.
-
Molecular pharmacology of P2Y-receptors.Naunyn Schmiedebergs Arch Pharmacol. 2000 Nov;362(4-5):310-23. doi: 10.1007/s002100000310. Naunyn Schmiedebergs Arch Pharmacol. 2000. PMID: 11111826 Review.
Cited by
-
Coupling switch of P2Y-IP3 receptors mediates differential Ca(2+) signaling in human embryonic stem cells and derived cardiovascular progenitor cells.Purinergic Signal. 2016 Sep;12(3):465-78. doi: 10.1007/s11302-016-9512-9. Epub 2016 Apr 20. Purinergic Signal. 2016. PMID: 27098757 Free PMC article.
-
Synthesis, structure-activity relationships and biological evaluation of benzimidazole derived sulfonylurea analogues as a new class of antagonists of P2Y1 receptor.Front Pharmacol. 2023 May 26;14:1217315. doi: 10.3389/fphar.2023.1217315. eCollection 2023. Front Pharmacol. 2023. PMID: 37305545 Free PMC article.
-
Perivascular innervation: a multiplicity of roles in vasomotor control and myoendothelial signaling.Microcirculation. 2013 Apr;20(3):217-38. doi: 10.1111/micc.12035. Microcirculation. 2013. PMID: 23289720 Free PMC article. Review.
-
Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor.Br J Pharmacol. 2014 Jul;171(13):3298-312. doi: 10.1111/bph.12685. Br J Pharmacol. 2014. PMID: 24628114 Free PMC article.
-
Gene expression profiles of ion channels and receptors in mouse resistance arteries: Effects of cell type, vascular bed, and age.Microcirculation. 2018 May;25(4):e12452. doi: 10.1111/micc.12452. Microcirculation. 2018. PMID: 29577514 Free PMC article.
References
-
- Vallot O, Combettes L, Jourdon P, Inamo J, Marty I, Claret M, Lompre AM. Intracellular Ca2+ handling in vascular smooth muscle cells is affected by proliferation. Pflugers Arch. 2000;20:1225–1235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

