Architecture and regulation of negative-strand viral enzymatic machinery
- PMID: 22767259
- PMCID: PMC3495737
- DOI: 10.4161/rna.20345
Architecture and regulation of negative-strand viral enzymatic machinery
Abstract
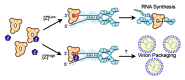
Negative-strand (NS) RNA viruses initiate infection with a unique polymerase complex that mediates both mRNA transcription and subsequent genomic RNA replication. For nearly all NS RNA viruses, distinct enzymatic domains catalyzing RNA polymerization and multiple steps of 5' mRNA cap formation are contained within a single large polymerase protein (L). While NS RNA viruses include a variety of emerging human and agricultural pathogens, the enzymatic machinery driving viral replication and gene expression remains poorly understood. Recent insights with Machupo virus and vesicular stomatitis virus have provided the first structural information of viral L proteins, and revealed how the various enzymatic domains are arranged into a conserved architecture shared by both segmented and nonsegmented NS RNA viruses. In vitro systems reconstituting RNA synthesis from purified components provide new tools to understand the viral replicative machinery, and demonstrate the arenavirus matrix protein regulates RNA synthesis by locking a polymerase-template complex. Inhibition of gene expression by the viral matrix protein is a distinctive feature also shared with influenza A virus and nonsegmented NS RNA viruses, possibly illuminating a conserved mechanism for coordination of viral transcription and polymerase packaging.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
