Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset
- PMID: 22767497
- PMCID: PMC3679641
- DOI: 10.1182/blood-2012-05-430470
Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset
Abstract
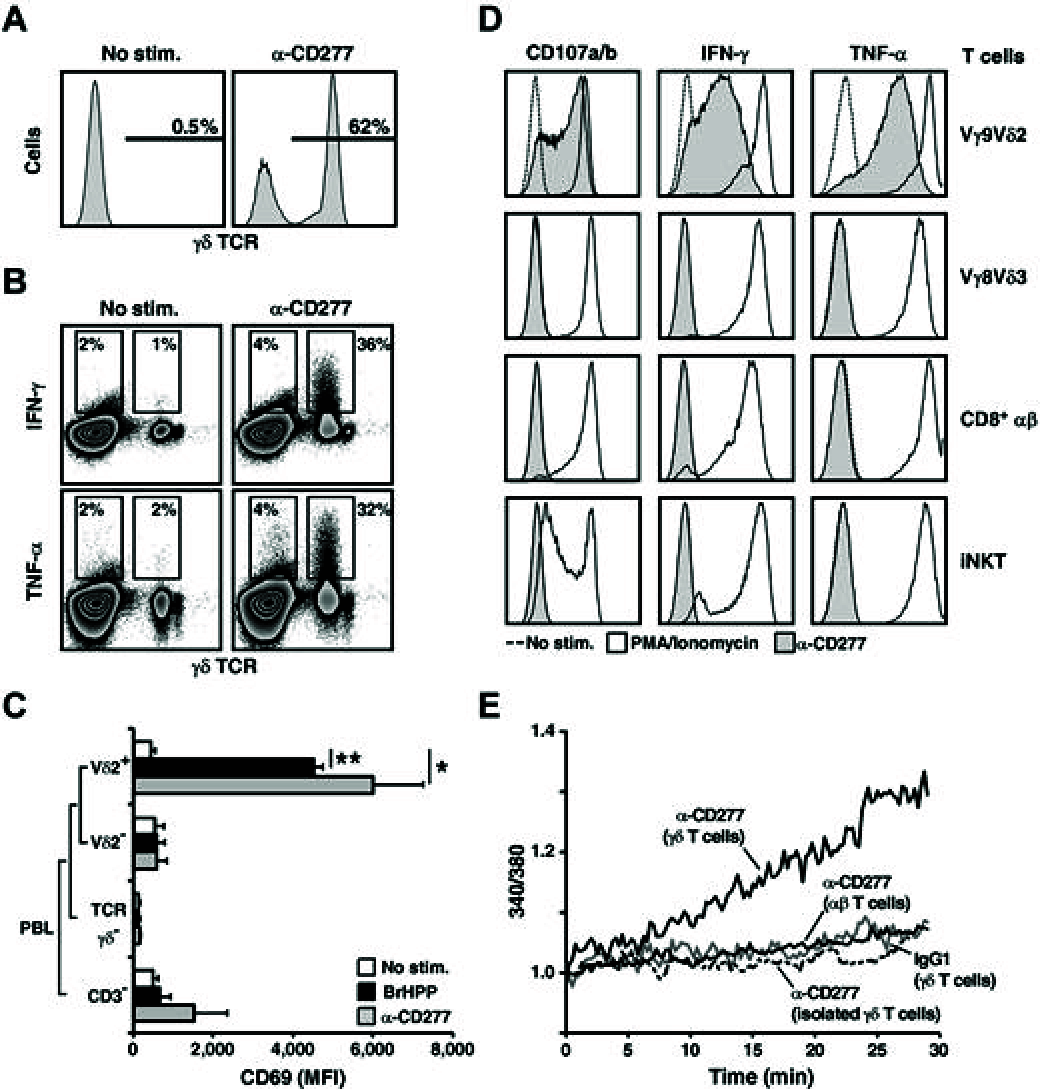
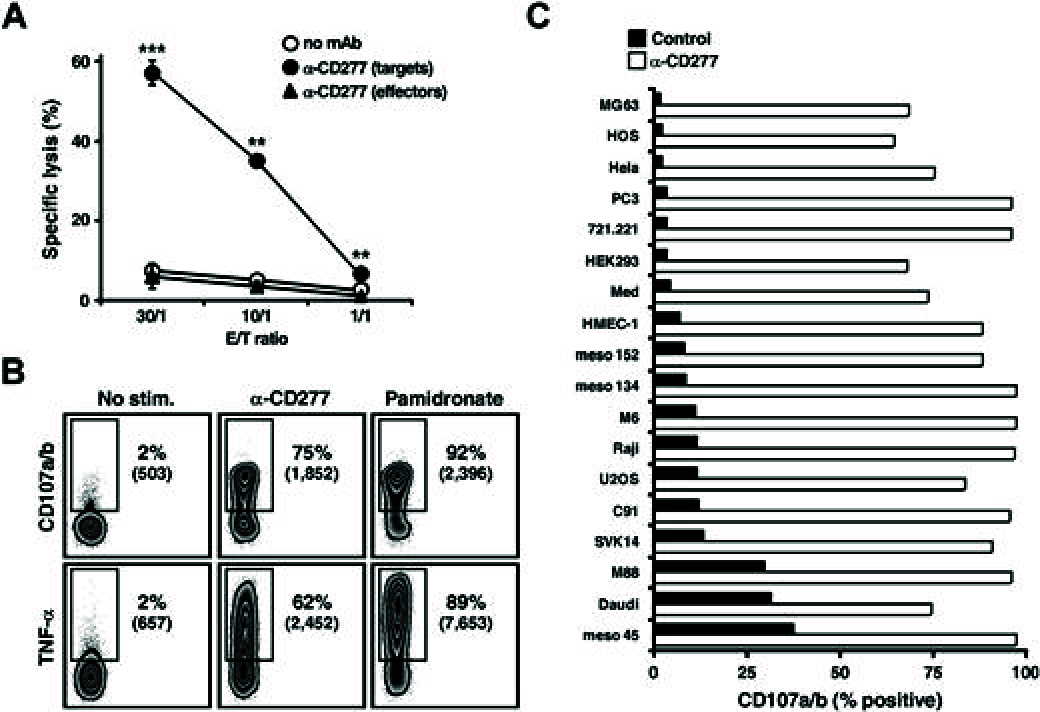
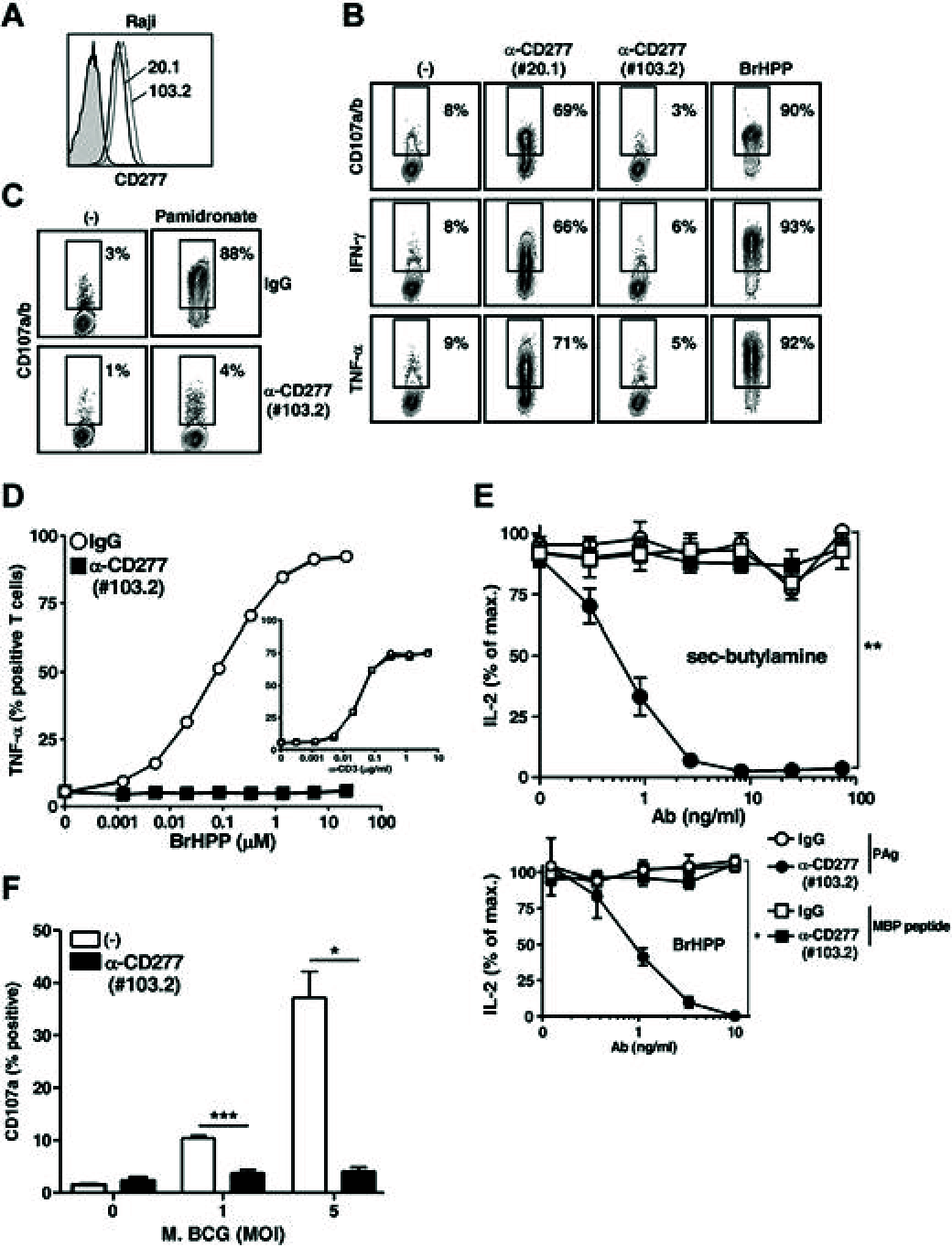
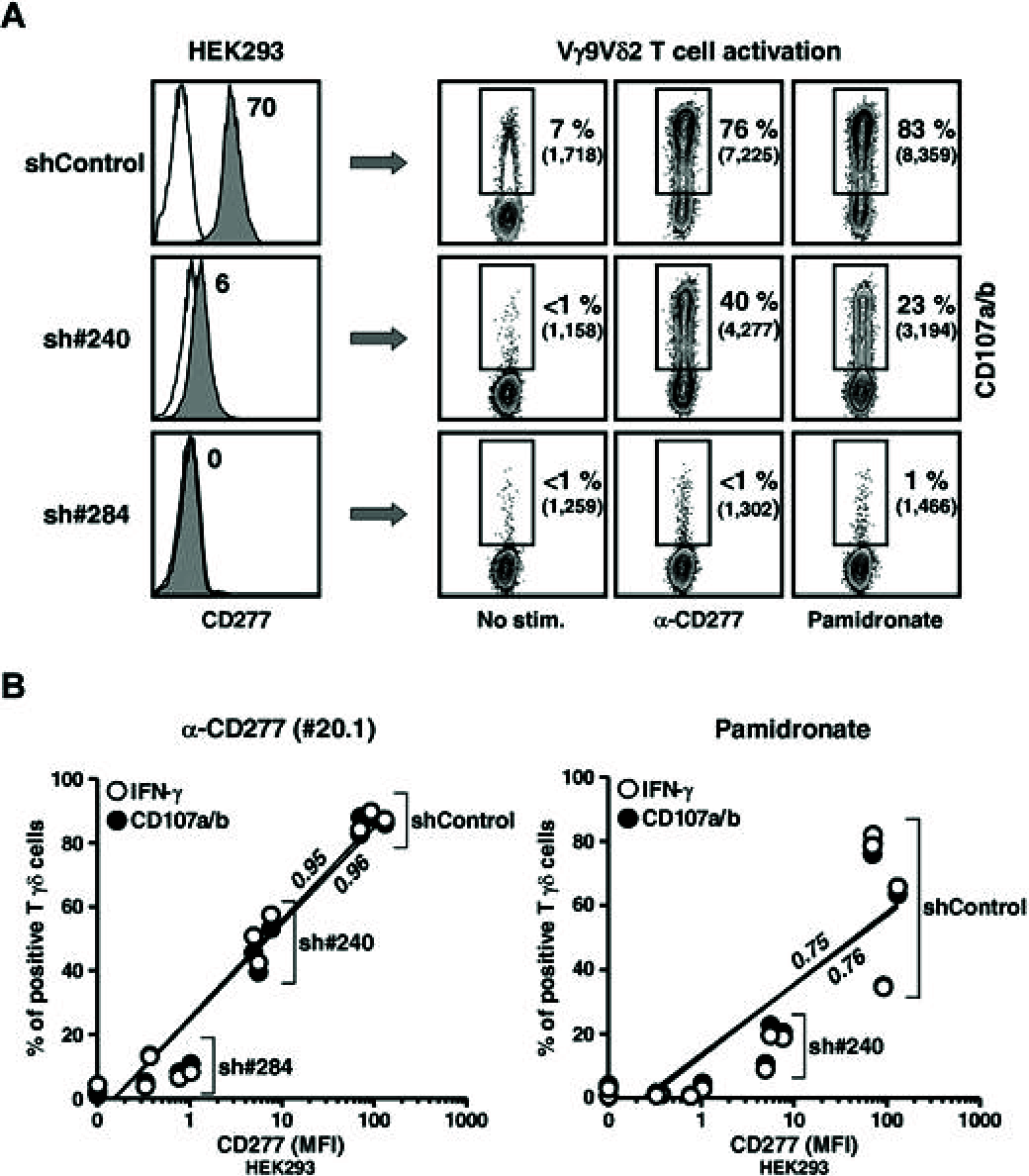
Human peripheral Vγ9Vδ2 T cells are activated by phosphorylated metabolites (phosphoagonists [PAg]) of the mammalian mevalonate or the microbial desoxyxylulose-phosphate pathways accumulated by infected or metabolically distressed cells. The underlying mechanisms are unknown. We show that treatment of nonsusceptible target cells with antibody 20.1 against CD277, a member of the extended B7 superfamily related to butyrophilin, mimics PAg-induced Vγ9Vδ2 T-cell activation and that the Vγ9Vδ2 T-cell receptor is implicated in this effect. Vγ9Vδ2 T-cell activation can be abrogated by exposing susceptible cells (tumor and mycobacteria-infected cells, or aminobisphosphonate-treated cells with up-regulated PAg levels) to antibody 103.2 against CD277. CD277 knockdown and domain-shuffling approaches confirm the key implication of the CD277 isoform BTN3A1 in PAg sensing by Vγ9Vδ2 T cells. Fluorescence recovery after photobleaching (FRAP) experiments support a causal link between intracellular PAg accumulation, decreased BTN3A1 membrane mobility, and ensuing Vγ9Vδ2 T-cell activation. This study demonstrates a novel role played by B7-like molecules in human γδ T-cell antigenic activation and paves the way for new strategies to improve the efficiency of immunotherapies using Vγ9Vδ2 T cells.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

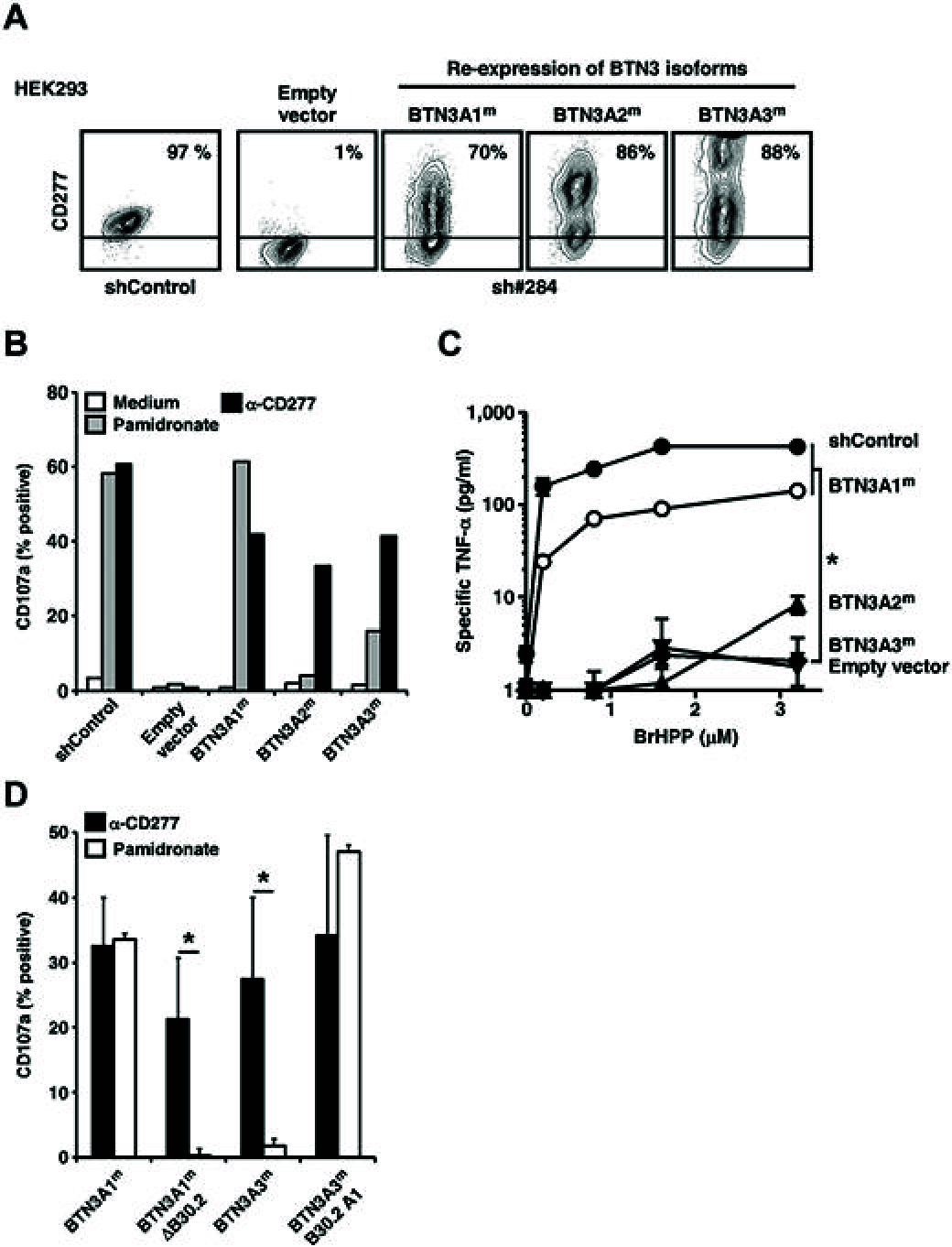
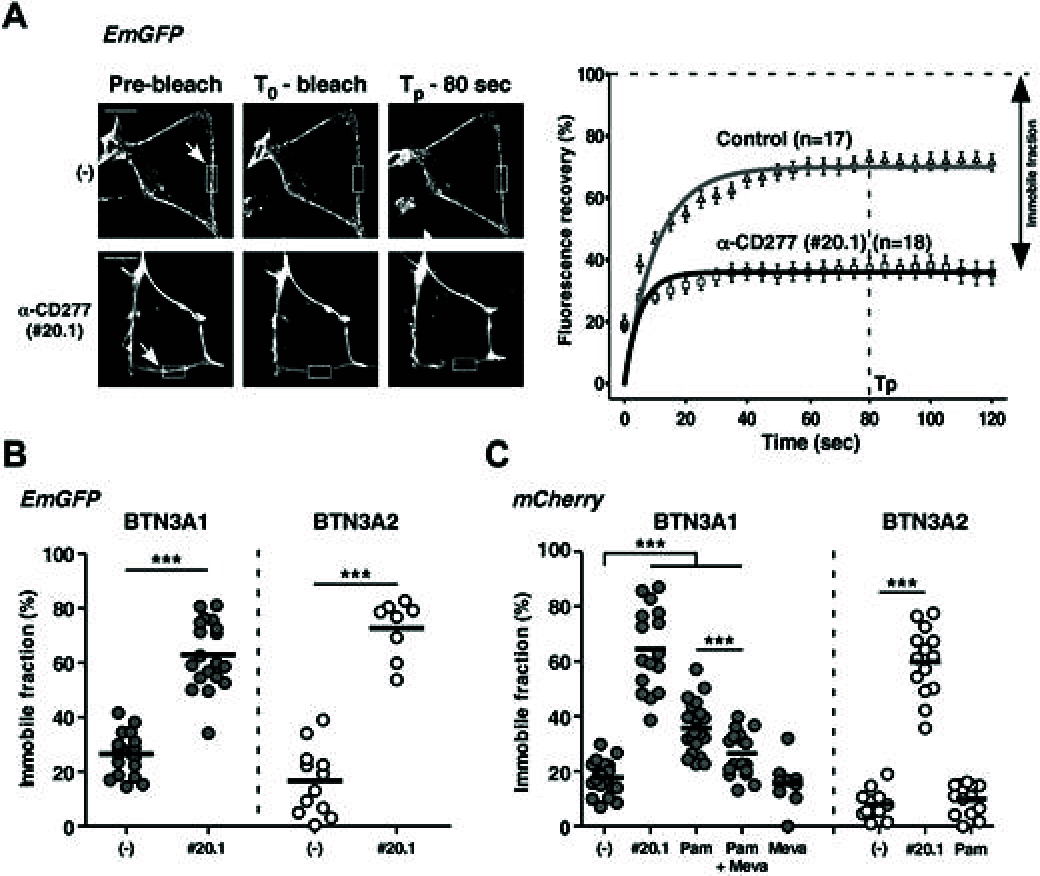
Comment in
-
CD277 takes the lead in human γδ T-cell activation.Blood. 2012 Sep 13;120(11):2159-61. doi: 10.1182/blood-2012-07-442731. Blood. 2012. PMID: 22977080 No abstract available.
References
-
- Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31(2):184–196. - PubMed
-
- Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10(7):467–478. - PubMed
-
- Chien YH, Konigshofer Y. Antigen recognition by gammadelta T cells. Immunol Rev. 2007;215:46–58. - PubMed
-
- Fournie JJ, Bonneville M. Stimulation of gamma delta T cells by phosphoantigens. Res Immunol. 1996;147(5):338–347. - PubMed
-
- Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol. 2006;18(5):539–546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

