Characterization of a multi-component anthrax vaccine designed to target the initial stages of infection as well as toxaemia
- PMID: 22767539
- PMCID: PMC3541767
- DOI: 10.1099/jmm.0.045393-0
Characterization of a multi-component anthrax vaccine designed to target the initial stages of infection as well as toxaemia
Abstract
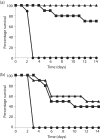
Current vaccine approaches to combat anthrax are effective; however, they target only a single protein [the protective antigen (PA) toxin component] that is produced after spore germination. PA production is subsequently increased during later vegetative cell proliferation. Accordingly, several aspects of the vaccine strategy could be improved. The inclusion of spore-specific antigens with PA could potentially induce protection to initial stages of the disease. Moreover, adding other epitopes to the current vaccine strategy will decrease the likelihood of encountering a strain of Bacillus anthracis (emerging or engineered) that is refractory to the vaccine. Adding recombinant spore-surface antigens (e.g. BclA, ExsFA/BxpB and p5303) to PA has been shown to augment protection afforded by the latter using a challenge model employing immunosuppressed mice challenged with spores derived from the attenuated Sterne strain of B. anthracis. This report demonstrated similar augmentation utilizing guinea pigs or mice challenged with spores of the fully virulent Ames strain or a non-toxigenic but encapsulated ΔAmes strain of B. anthracis, respectively. Additionally, it was shown that immune interference did not occur if optimal amounts of antigen were administered. By administering the toxin and spore-based immunogens simultaneously, a significant adjuvant effect was also observed in some cases. Thus, these data further support the inclusion of recombinant spore antigens in next-generation anthrax vaccine strategies.
Figures
References
-
- Beedham R. J., Turnbull P. C., Williamson E. D. (2001). Passive transfer of protection against Bacillus anthracis infection in a murine model. Vaccine 19, 4409–4416 - PubMed
-
- Bielinska A. U., Janczak K. W., Landers J. J., Makidon P., Sower L. E., Peterson J. W., Baker J. R., Jr (2007). Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge. Infect Immun 75, 4020–4029 10.1128/IAI.00070-07 - DOI - PMC - PubMed
-
- Boyaka P. N., Tafaro A., Fischer R., Leppla S. H., Fujihashi K., McGhee J. R. (2003). Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J Immunol 170, 5636–5643 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

