Vibrio parahaemolyticus ExsE is requisite for initial adhesion and subsequent type III secretion system 1-dependent autophagy in HeLa cells
- PMID: 22767546
- PMCID: PMC3542814
- DOI: 10.1099/mic.0.059931-0
Vibrio parahaemolyticus ExsE is requisite for initial adhesion and subsequent type III secretion system 1-dependent autophagy in HeLa cells
Abstract
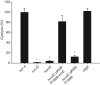
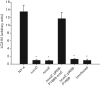
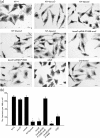
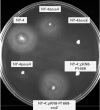
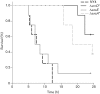
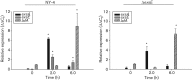
Vibrio parahaemolyticus pandemic serotype O3 : K6 causes acute gastroenteritis, wound infections and septicaemia in humans. This organism encodes two type III secretion systems (T3SS1 and T3SS2); host-cell cytotoxicity has been attributed to T3SS1. Synthesis and secretion of T3SS1 proteins is positively regulated by ExsA, which is presumptively regulated by the ExsCDE pathway, similar to Pseudomonas aeruginosa. Herein we deleted the putative exsE from V. parahaemolyticus and found constitutive expression of the T3SS1 in broth culture as expected. More importantly, however, in a cell culture model, the ΔexsE strain was unable to induce cytotoxicity, as measured by release of lactate dehydrogenase (LDH), or autophagy, as measured by LC3 conversion. This is markedly different from P. aeruginosa, where deletion of exsE has no effect on host-cell cytolysis. Swarming and cytoadhesion were reduced for the deletion mutant and could be recovered along with T3SS1-induced HeLa cell cytotoxicity by in cis expression of exsE in the ΔexsE strain. Loss of adhesion and swarming motility was associated with the loss of flagella biogenesis in the exsE-deficient strain. Mouse mortality was unaffected by the deletion of exsE compared with a wild-type control, suggesting that additional adhesins are important for intoxication in vivo. Based on these data, we conclude that ExsE contributes to the negative regulation of T3SS1 and, in addition, contributes to regulation of an adherence phenotype that is requisite for translocation of effector proteins into HeLa cells.
Figures

Similar articles
-
Vp1659 is a Vibrio parahaemolyticus type III secretion system 1 protein that contributes to translocation of effector proteins needed to induce cytolysis, autophagy, and disruption of actin structure in HeLa cells.J Bacteriol. 2010 Jul;192(13):3491-502. doi: 10.1128/JB.01493-09. Epub 2010 Apr 23. J Bacteriol. 2010. PMID: 20418402 Free PMC article.
-
Development of two animal models to study the function of Vibrio parahaemolyticus type III secretion systems.Infect Immun. 2010 Nov;78(11):4551-9. doi: 10.1128/IAI.00461-10. Epub 2010 Sep 7. Infect Immun. 2010. PMID: 20823199 Free PMC article.
-
Structural and regulatory mutations in Vibrio parahaemolyticus type III secretion systems display variable effects on virulence.FEMS Microbiol Lett. 2014 Dec;361(2):107-14. doi: 10.1111/1574-6968.12619. Epub 2014 Oct 31. FEMS Microbiol Lett. 2014. PMID: 25288215 Free PMC article.
-
[Type III secretion system of Vibrio parahaemolyticus--a review].Wei Sheng Wu Xue Bao. 2009 Jul;49(7):848-52. Wei Sheng Wu Xue Bao. 2009. PMID: 19873746 Review. Chinese.
-
The role of type III secretion system 2 in Vibrio parahaemolyticus pathogenicity.J Microbiol. 2012 Oct;50(5):719-25. doi: 10.1007/s12275-012-2550-2. Epub 2012 Nov 4. J Microbiol. 2012. PMID: 23124738 Review.
Cited by
-
Characterization of the RpoN regulon reveals the regulation of motility, T6SS2 and metabolism in Vibrio parahaemolyticus.Front Microbiol. 2022 Dec 22;13:1025960. doi: 10.3389/fmicb.2022.1025960. eCollection 2022. Front Microbiol. 2022. PMID: 36620062 Free PMC article.
-
Role of the TPR family protein VPA1365 in regulating type III secretion system 2 and virulence in Vibrio parahaemolyticus.Appl Environ Microbiol. 2025 Apr 23;91(4):e0220124. doi: 10.1128/aem.02201-24. Epub 2025 Mar 25. Appl Environ Microbiol. 2025. PMID: 40130841 Free PMC article.
-
Type III Secretion 1 Effector Gene Diversity Among Vibrio Isolates From Coastal Areas in China.Front Cell Infect Microbiol. 2020 Jun 18;10:301. doi: 10.3389/fcimb.2020.00301. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32637366 Free PMC article.
-
A Novel Transcription Factor VPA0041 Was Identified to Regulate the Swarming Motility in Vibrio parahaemolyticus.Pathogens. 2022 Apr 10;11(4):453. doi: 10.3390/pathogens11040453. Pathogens. 2022. PMID: 35456128 Free PMC article.
-
Regulatory actions of ToxR and CalR on their own genes and type III secretion system 1 in Vibrio parahaemolyticus.Oncotarget. 2017 Jul 22;8(39):65809-65822. doi: 10.18632/oncotarget.19498. eCollection 2017 Sep 12. Oncotarget. 2017. PMID: 29029474 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

