Aurora B is regulated by the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway and is a valuable potential target in melanoma cells
- PMID: 22767597
- PMCID: PMC3436153
- DOI: 10.1074/jbc.M112.371682
Aurora B is regulated by the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway and is a valuable potential target in melanoma cells
Abstract
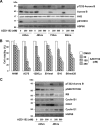
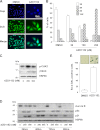
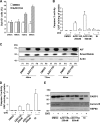
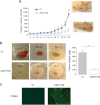
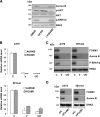
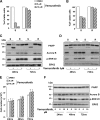
Metastatic melanoma is a deadly skin cancer and is resistant to almost all existing treatment. Vemurafenib, which targets the BRAFV600E mutation, is one of the drugs that improves patient outcome, but the patients next develop secondary resistance and a return to cancer. Thus, new therapeutic strategies are needed to treat melanomas and to increase the duration of v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) inhibitor response. The ERK pathway controls cell proliferation, and Aurora B plays a pivotal role in cell division. Here, we confirm that Aurora B is highly expressed in metastatic melanoma cells and that Aurora B inhibition triggers both senescence-like phenotypes and cell death in melanoma cells. Furthermore, we show that the BRAF/ERK axis controls Aurora B expression at the transcriptional level, likely through the transcription factor FOXM1. Our results provide insight into the mechanism of Aurora B regulation and the first molecular basis of Aurora B regulation in melanoma cells. The inhibition of Aurora B expression that we observed in vemurafenib-sensitive melanoma cells was rescued in cells resistant to this drug. Consistently, these latter cells remain sensitive to the effect of the Aurora B inhibitor. Noteworthy, wild-type BRAF melanoma cells are also sensitive to Aurora B inhibition. Collectively, our findings, showing that Aurora B is a potential target in melanoma cells, particularly in those vemurafenib-resistant, may open new avenues to improve the treatment of metastatic melanoma.
Figures


References
-
- Hodi F. S., O'Day S. J., McDermott D. F., Weber R. W., Sosman J. A., Haanen J. B., Gonzalez R., Robert C., Schadendorf D., Hassel J. C., Akerley W., van den Eertwegh A. J., Lutzky J., Lorigan P., Vaubel J. M., Linette G. P., Hogg D., Ottensmeier C. H., Lebbé C., Peschel C., Quirt I., Clark J. I., Wolchok J. D., Weber J. S., Tian J., Yellin M. J., Nichol G. M., Hoos A., Urba W. J. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 - PMC - PubMed
-
- Davies H., Bignell G. R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M. J., Bottomley W., Davis N., Dicks E., Ewing R., Floyd Y., Gray K., Hall S., Hawes R., Hughes J., Kosmidou V., Menzies A., Mould C., Parker A., Stevens C., Watt S., Hooper S., Wilson R., Jayatilake H., Gusterson B. A., Cooper C., Shipley J., Hargrave D., Pritchard-Jones K., Maitland N., Chenevix-Trench G., Riggins G. J., Bigner D. D., Palmieri G., Cossu A., Flanagan A., Nicholson A., Ho J. W., Leung S. Y., Yuen S. T., Weber B. L., Seigler H. F., Darrow T. L., Paterson H., Marais R., Marshall C. J., Wooster R., Stratton M. R., Futreal P. A. (2002) Mutations of the BRAF gene in human cancer. Nature 417, 949–954 - PubMed
-
- Chapman P. B., Hauschild A., Robert C., Haanen J. B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., Hogg D., Lorigan P., Lebbe C., Jouary T., Schadendorf D., Ribas A., O'Day S. J., Sosman J. A., Kirkwood J. M., Eggermont A. M., Dreno B., Nolop K., Li J., Nelson B., Hou J., Lee R. J., Flaherty K. T., McArthur G. A. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 - PMC - PubMed
-
- Ruchaud S., Carmena M., Earnshaw W. C. (2007) Chromosomal passengers. Conducting cell division. Nat. Rev. Mol. Cell Biol. 8, 798–812 - PubMed
-
- Pirker C., Lötsch D., Spiegl-Kreinecker S., Jantscher F., Sutterlüty H., Micksche M., Grusch M., Berger W. (2010) Response of experimental malignant melanoma models to the pan-Aurora kinase inhibitor VE-465. Exp. Dermatol. 19, 1040–1047 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

