Role of twin Cys-Xaa9-Cys motif cysteines in mitochondrial import of the cytochrome C oxidase biogenesis factor Cmc1
- PMID: 22767599
- PMCID: PMC3438957
- DOI: 10.1074/jbc.M112.383562
Role of twin Cys-Xaa9-Cys motif cysteines in mitochondrial import of the cytochrome C oxidase biogenesis factor Cmc1
Abstract
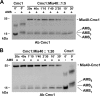
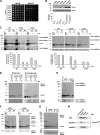
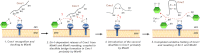
The Mia40 import pathway facilitates the import and oxidative folding of cysteine-rich protein substrates into the mitochondrial intermembrane space. Here we describe the in vitro and in organello oxidative folding of Cmc1, a twin CX(9)C-containing substrate, which contains an unpaired cysteine. In vitro, Cmc1 can be oxidized by the import receptor Mia40 alone when in excess or at a lower rate by only the sulfhydryl oxidase Erv1. However, physiological and efficient Cmc1 oxidation requires Erv1 and Mia40. Cmc1 forms a stable intermediate with Mia40 and is released from this interaction in the presence of Erv1. The three proteins are shown to form a ternary complex in mitochondria. Our results suggest that this mechanism facilitates efficient formation of multiple disulfides and prevents the formation of non-native disulfide bonds.
Figures





Similar articles
-
Mia40 Protein Serves as an Electron Sink in the Mia40-Erv1 Import Pathway.J Biol Chem. 2015 Aug 21;290(34):20804-20814. doi: 10.1074/jbc.M115.669440. Epub 2015 Jun 17. J Biol Chem. 2015. PMID: 26085103 Free PMC article.
-
Structural and functional roles of the conserved cysteine residues of the redox-regulated import receptor Mia40 in the intermembrane space of mitochondria.J Biol Chem. 2009 Jan 16;284(3):1353-63. doi: 10.1074/jbc.M805035200. Epub 2008 Nov 14. J Biol Chem. 2009. PMID: 19011240
-
Mitochondrial Ccs1 contains a structural disulfide bond crucial for the import of this unconventional substrate by the disulfide relay system.Mol Biol Cell. 2011 Oct;22(20):3758-67. doi: 10.1091/mbc.E11-04-0296. Epub 2011 Aug 24. Mol Biol Cell. 2011. PMID: 21865601 Free PMC article.
-
A disulfide relay system in mitochondria.Cell. 2005 Jul 1;121(7):965-7. doi: 10.1016/j.cell.2005.06.019. Cell. 2005. PMID: 15989945 Review.
-
Structural basis for the disulfide relay system in the mitochondrial intermembrane space.Antioxid Redox Signal. 2010 Nov 1;13(9):1359-73. doi: 10.1089/ars.2010.3099. Antioxid Redox Signal. 2010. PMID: 20136511 Review.
Cited by
-
Oxidative folding in the mitochondrial intermembrane space in human health and disease.Int J Mol Sci. 2013 Jan 30;14(2):2916-27. doi: 10.3390/ijms14022916. Int J Mol Sci. 2013. PMID: 23364613 Free PMC article.
-
Mia40 Protein Serves as an Electron Sink in the Mia40-Erv1 Import Pathway.J Biol Chem. 2015 Aug 21;290(34):20804-20814. doi: 10.1074/jbc.M115.669440. Epub 2015 Jun 17. J Biol Chem. 2015. PMID: 26085103 Free PMC article.
-
Erv1 of Arabidopsis thaliana can directly oxidize mitochondrial intermembrane space proteins in the absence of redox-active Mia40.BMC Biol. 2017 Nov 8;15(1):106. doi: 10.1186/s12915-017-0445-8. BMC Biol. 2017. PMID: 29117860 Free PMC article.
-
MIA40 suppresses cell death induced by apoptosis-inducing factor 1.EMBO Rep. 2025 Apr;26(7):1835-1862. doi: 10.1038/s44319-025-00406-8. Epub 2025 Mar 7. EMBO Rep. 2025. PMID: 40055465 Free PMC article.
-
Redox-Mediated Regulation of Mitochondrial Biogenesis, Dynamics, and Respiratory Chain Assembly in Yeast and Human Cells.Front Cell Dev Biol. 2021 Sep 7;9:720656. doi: 10.3389/fcell.2021.720656. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34557489 Free PMC article. Review.
References
-
- Endo T., Yamano K. (2009) Multiple pathways for mitochondrial protein traffic. Biol. Chem. 390, 723–730 - PubMed
-
- Naoé M., Ohwa Y., Ishikawa D., Ohshima C., Nishikawa S., Yamamoto H., Endo T. (2004) Identification of Tim40 that mediates protein sorting to the mitochondrial intermembrane space. J. Biol. Chem. 279, 47815–47821 - PubMed
-
- Mesecke N., Terziyska N., Kozany C., Baumann F., Neupert W., Hell K., Herrmann J. M. (2005) A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 121, 1059–1069 - PubMed
-
- Herrmann J. M., Kauff F., Neuhaus H. E. (2009) Thiol oxidation in bacteria, mitochondria and chloroplasts: common principles but three unrelated machineries? Biochim. Biophys. Acta 1793, 71–77 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

