Role of twin Cys-Xaa9-Cys motif cysteines in mitochondrial import of the cytochrome C oxidase biogenesis factor Cmc1
- PMID: 22767599
- PMCID: PMC3438957
- DOI: 10.1074/jbc.M112.383562
Role of twin Cys-Xaa9-Cys motif cysteines in mitochondrial import of the cytochrome C oxidase biogenesis factor Cmc1
Abstract
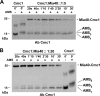
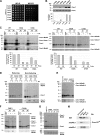
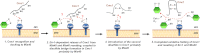
The Mia40 import pathway facilitates the import and oxidative folding of cysteine-rich protein substrates into the mitochondrial intermembrane space. Here we describe the in vitro and in organello oxidative folding of Cmc1, a twin CX(9)C-containing substrate, which contains an unpaired cysteine. In vitro, Cmc1 can be oxidized by the import receptor Mia40 alone when in excess or at a lower rate by only the sulfhydryl oxidase Erv1. However, physiological and efficient Cmc1 oxidation requires Erv1 and Mia40. Cmc1 forms a stable intermediate with Mia40 and is released from this interaction in the presence of Erv1. The three proteins are shown to form a ternary complex in mitochondria. Our results suggest that this mechanism facilitates efficient formation of multiple disulfides and prevents the formation of non-native disulfide bonds.
Figures





References
-
- Endo T., Yamano K. (2009) Multiple pathways for mitochondrial protein traffic. Biol. Chem. 390, 723–730 - PubMed
-
- Naoé M., Ohwa Y., Ishikawa D., Ohshima C., Nishikawa S., Yamamoto H., Endo T. (2004) Identification of Tim40 that mediates protein sorting to the mitochondrial intermembrane space. J. Biol. Chem. 279, 47815–47821 - PubMed
-
- Mesecke N., Terziyska N., Kozany C., Baumann F., Neupert W., Hell K., Herrmann J. M. (2005) A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 121, 1059–1069 - PubMed
-
- Herrmann J. M., Kauff F., Neuhaus H. E. (2009) Thiol oxidation in bacteria, mitochondria and chloroplasts: common principles but three unrelated machineries? Biochim. Biophys. Acta 1793, 71–77 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

