Specific targeting of the metallophosphoesterase YkuE to the bacillus cell wall requires the twin-arginine translocation system
- PMID: 22767609
- PMCID: PMC3436186
- DOI: 10.1074/jbc.M112.378190
Specific targeting of the metallophosphoesterase YkuE to the bacillus cell wall requires the twin-arginine translocation system
Abstract
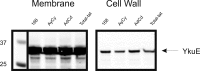
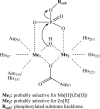
The twin-arginine translocation (Tat) pathway is dedicated to the transport of fully folded proteins across the cytoplasmic membranes of many bacteria and the chloroplast thylakoidal membrane. Accordingly, Tat-dependently translocated proteins are known to be delivered to the periplasm of Gram-negative bacteria, the growth medium of Gram-positive bacteria, and the thylakoid lumen. Here, we present the first example of a protein, YkuE of Bacillus subtilis, that is specifically targeted by the Tat pathway to the cell wall of a Gram-positive bacterium. The cell wall binding of YkuE is facilitated by electrostatic interactions. Interestingly, under particular conditions, YkuE can also be targeted to the cell wall in a Tat-independent manner. The biological function of YkuE was so far unknown. Our present studies show that YkuE is a metal-dependent phosphoesterase that preferentially binds manganese and zinc.
Figures






Similar articles
-
The Twin-Arginine Pathway for Protein Secretion.EcoSal Plus. 2019 Jun;8(2):10.1128/ecosalplus.ESP-0040-2018. doi: 10.1128/ecosalplus.ESP-0040-2018. EcoSal Plus. 2019. PMID: 31215506 Free PMC article. Review.
-
Two minimal Tat translocases in Bacillus.Mol Microbiol. 2004 Dec;54(5):1319-25. doi: 10.1111/j.1365-2958.2004.04341.x. Mol Microbiol. 2004. PMID: 15554971
-
The Tat system of Gram-positive bacteria.Biochim Biophys Acta. 2014 Aug;1843(8):1698-706. doi: 10.1016/j.bbamcr.2013.10.008. Epub 2013 Oct 16. Biochim Biophys Acta. 2014. PMID: 24140208 Review.
-
Double trouble: Bacillus depends on a functional Tat machinery to avoid severe oxidative stress and starvation upon entry into a NaCl-depleted environment.Biochim Biophys Acta Mol Cell Res. 2021 Feb;1868(2):118914. doi: 10.1016/j.bbamcr.2020.118914. Epub 2020 Nov 25. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 33245978
-
Functional association of the stress-responsive LiaH protein and the minimal TatAyCy protein translocase in Bacillus subtilis.Biochim Biophys Acta Mol Cell Res. 2020 Aug;1867(8):118719. doi: 10.1016/j.bbamcr.2020.118719. Epub 2020 Apr 14. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32302670
Cited by
-
The Twin-Arginine Pathway for Protein Secretion.EcoSal Plus. 2019 Jun;8(2):10.1128/ecosalplus.ESP-0040-2018. doi: 10.1128/ecosalplus.ESP-0040-2018. EcoSal Plus. 2019. PMID: 31215506 Free PMC article. Review.
-
A unifying mechanism for the biogenesis of membrane proteins co-operatively integrated by the Sec and Tat pathways.Elife. 2017 May 17;6:e26577. doi: 10.7554/eLife.26577. Elife. 2017. PMID: 28513434 Free PMC article.
-
Co-factor insertion and disulfide bond requirements for twin-arginine translocase-dependent export of the Bacillus subtilis Rieske protein QcrA.J Biol Chem. 2014 May 9;289(19):13124-31. doi: 10.1074/jbc.M113.529677. Epub 2014 Mar 20. J Biol Chem. 2014. PMID: 24652282 Free PMC article.
-
Transport of Folded Proteins by the Tat System.Protein J. 2019 Aug;38(4):377-388. doi: 10.1007/s10930-019-09859-y. Protein J. 2019. PMID: 31401776 Free PMC article. Review.
-
Degradation of the twin-arginine translocation substrate YwbN by extracytoplasmic proteases of Bacillus subtilis.Appl Environ Microbiol. 2012 Nov;78(21):7801-4. doi: 10.1128/AEM.02023-12. Epub 2012 Aug 24. Appl Environ Microbiol. 2012. PMID: 22923395 Free PMC article.
References
-
- Robinson C., Matos C. F., Beck D., Ren C., Lawrence J., Vasisht N., Mendel S. (2011) Transport and proofreading of proteins by the twin-arginine translocation (Tat) system in bacteria. Biochim. Biophys. Acta 1808, 876–884 - PubMed
-
- Robinson C, Bolhuis A. (2004) Tat-dependent protein targeting in prokaryotes and chloroplasts. Biochim. Biophys. Acta 1694, 135–147 - PubMed
-
- Tjalsma H., Antelmann H., Jongbloed J. D., Braun P. G., Darmon E., Dorenbos R., Dubois J. Y., Westers H., Zanen G., Quax W. J., Kuipers O. P., Bron S., Hecker M., van Dijl J. M. (2004) Proteomics of protein secretion by Bacillus subtilis. Separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68, 207–233 - PMC - PubMed
-
- Palmer T., Sargent F., Berks B. C. (2005) Export of complex cofactor-containing proteins by the bacterial Tat pathway. Trends Microbiol. 13, 175–180 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

