Country, sex, EDSS change and therapy choice independently predict treatment discontinuation in multiple sclerosis and clinically isolated syndrome
- PMID: 22768046
- PMCID: PMC3387159
- DOI: 10.1371/journal.pone.0038661
Country, sex, EDSS change and therapy choice independently predict treatment discontinuation in multiple sclerosis and clinically isolated syndrome
Abstract
Objectives: We conducted a prospective study, MSBASIS, to assess factors leading to first treatment discontinuation in patients with a clinically isolated syndrome (CIS) and early relapsing-remitting multiple sclerosis (RRMS).
Methods: The MSBASIS Study, conducted by MSBase Study Group members, enrols patients seen from CIS onset, reporting baseline demographics, cerebral magnetic resonance imaging (MRI) features and Expanded Disability Status Scale (EDSS) scores. Follow-up visits report relapses, EDSS scores, and the start and end dates of MS-specific therapies. We performed a multivariable survival analysis to determine factors within this dataset that predict first treatment discontinuation.
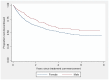
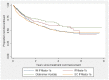
Results: A total of 2314 CIS patients from 44 centres were followed for a median of 2.7 years, during which time 1247 commenced immunomodulatory drug (IMD) treatment. Ninety percent initiated IMD after a diagnosis of MS was confirmed, and 10% while still in CIS status. Over 40% of these patients stopped their first IMD during the observation period. Females were more likely to cease medication than males (HR 1.36, p = 0.003). Patients treated in Australia were twice as likely to cease their first IMD than patients treated in Spain (HR 1.98, p = 0.001). Increasing EDSS was associated with higher rate of IMD cessation (HR 1.21 per EDSS unit, p<0.001), and intramuscular interferon-β-1a (HR 1.38, p = 0.028) and subcutaneous interferon-β-1a (HR 1.45, p = 0.012) had higher rates of discontinuation than glatiramer acetate, although this varied widely in different countries. Onset cerebral MRI features, age, time to treatment initiation or relapse on treatment were not associated with IMD cessation.
Conclusion: In this multivariable survival analysis, female sex, country of residence, EDSS change and IMD choice independently predicted time to first IMD cessation.
Conflict of interest statement
Figures
References
-
- Filippi M, Rovaris M, Inglese M, Barkhof F, De Stefano N, et al. Interferon β-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:1489–1496. - PubMed
-
- Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle, et al. Intramuscular interferon β-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med. 2000;343:898–904. - PubMed
-
- Kinkel RP, Kollman C, O’Connor P, Murray TJ, Simon J, et al. IM interferon β-1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology. 2006;66:678–684. - PubMed
-
- Comi G, Filippi M, Barkhof F, Durelli L, Edan G, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Early Treatment of Multiple Sclerosis Study Group. Lancet. 2001;357:1576–1582. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

