Prevention of birch pollen-related food allergy by mucosal treatment with multi-allergen-chimers in mice
- PMID: 22768077
- PMCID: PMC3387141
- DOI: 10.1371/journal.pone.0039409
Prevention of birch pollen-related food allergy by mucosal treatment with multi-allergen-chimers in mice
Abstract
Background: Among birch pollen allergic patients up to 70% develop allergic reactions to Bet v 1-homologue food allergens such as Api g 1 (celery) or Dau c 1 (carrot), termed as birch pollen-related food allergy. In most cases, specific immunotherapy with birch pollen extracts does not reduce allergic symptoms to the homologue food allergens. We therefore genetically engineered a multi-allergen chimer and tested if mucosal treatment with this construct could represent a novel approach for prevention of birch pollen-related food allergy.
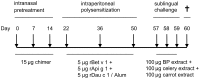
Methodology: BALB/c mice were poly-sensitized with a mixture of Bet v 1, Api g 1 and Dau c 1 followed by a sublingual challenge with carrot, celery and birch pollen extracts. For prevention of allergy sensitization an allergen chimer composed of immunodominant T cell epitopes of Api g 1 and Dau c 1 linked to the whole Bet v 1 allergen, was intranasally applied prior to sensitization.
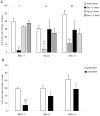
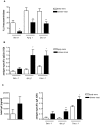
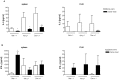
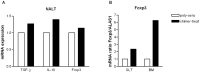
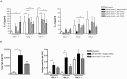
Results: Intranasal pretreatment with the allergen chimer led to significantly decreased antigen-specific IgE-dependent β-hexosaminidase release, but enhanced allergen-specific IgG2a and IgA antibodies. Accordingly, IL-4 levels in spleen cell cultures and IL-5 levels in restimulated spleen and cervical lymph node cell cultures were markedly reduced, while IFN-γ levels were increased. Immunomodulation was associated with increased IL-10, TGF-β and Foxp3 mRNA levels in NALT and Foxp3 in oral mucosal tissues. Treatment with anti-TGF-β, anti-IL10R or anti-CD25 antibodies abrogated the suppression of allergic responses induced by the chimer.
Conclusion: Our results indicate that mucosal application of the allergen chimer led to decreased Th2 immune responses against Bet v 1 and its homologue food allergens Api g 1 and Dau c 1 by regulatory and Th1-biased immune responses. These data suggest that mucosal treatment with a multi-allergen vaccine could be a promising treatment strategy to prevent birch pollen-related food allergy.
Conflict of interest statement
Figures


References
-
- Bohle B. The impact of pollen-related food allergens on pollen allergy. Allergy. 2007;62:3–10. - PubMed
-
- Bucher X, Pichler WJ, Dahinden CA, Helbling A. Effect of tree pollen specific, subcutaneous immunotherapy on the oral allergy syndrome to apple and hazelnut. Allergy. 2004;59:1272–1276. - PubMed
-
- Hoffmann-Sommergruber K. Plant allergens and pathogenesis-related proteins. What do they have in common? Int Arch Allergy Immunol. 2000;122:155–166. - PubMed
-
- Steinman H. Oral allergy syndrome - whats new? Current Allergy & Clinical Immunology 22. 2009.
-
- Geroldinger-Simic M, Zelniker T, Aberer W, Ebner C, Egger C, et al. Birch pollen-related food allergy: clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J Allergy Clin Immunol 127: 616–622 e611. 2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

