Myocardial autophagy after severe burn in rats
- PMID: 22768082
- PMCID: PMC3387177
- DOI: 10.1371/journal.pone.0039488
Myocardial autophagy after severe burn in rats
Abstract
Background: Autophagy plays a major role in myocardial ischemia and hypoxia injury. The present study investigated the effects of autophagy on cardiac dysfunction in rats after severe burn.
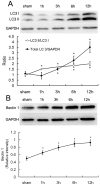
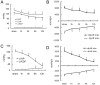
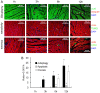
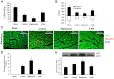
Methods: Protein expression of the autophagy markers LC3 and Beclin 1 were determined at 0, 1, 3, 6, and 12 h post-burn in Sprague Dawley rats subjected to 30% total body surface area 3rd degree burns. Autophagic, apoptotic, and oncotic cell death were evaluated in the myocardium at each time point by immunofluorescence. Changes of cardiac function were measured in a Langendorff model of isolated heart at 6 h post-burn, and the autophagic response was measured following activation by Rapamycin and inhibition by 3-methyladenine (3-MA). The angiotensin converting enzyme inhibitor enalaprilat, the angiotensin receptor I blocker losartan, and the reactive oxygen species inhibitor diphenylene iodonium (DPI) were also applied to the ex vivo heart model to examine the roles of these factors in post-burn cardiac function.
Results: Autophagic cell death was first observed in the myocardium at 3 h post-burn, occurring in 0.008 ± 0.001% of total cardiomyocytes, and continued to increase to a level of 0.022 ± 0.005% by 12 h post-burn. No autophagic cell death was observed in control hearts. Compared with apoptosis, autophagic cell death occurred earlier and in larger quantities. Rapamycin enhanced autophagy and decreased cardiac function in isolated hearts 6 h post-burn, while 3-MA exerted the opposite response. Enalaprilat, losartan, and DPI all inhibited autophagy and enhanced heart function.
Conclusion: Myocardial autophagy is enhanced in severe burns and autophagic cell death occurred early at 3 h post-burn, which may contribute to post-burn cardiac dysfunction. Angiotensin II and reactive oxygen species may play important roles in this process by regulating cell signaling transduction.
Conflict of interest statement
Figures
Similar articles
-
Berbamine postconditioning protects the heart from ischemia/reperfusion injury through modulation of autophagy.Cell Death Dis. 2017 Feb 2;8(2):e2577. doi: 10.1038/cddis.2017.7. Cell Death Dis. 2017. PMID: 28151484 Free PMC article.
-
The roles of autophagy and apoptosis in burn wound progression in rats.Burns. 2013 Dec;39(8):1551-6. doi: 10.1016/j.burns.2013.04.018. Epub 2013 Jun 14. Burns. 2013. PMID: 23751274
-
Role of autophagy and its molecular mechanisms in mice intestinal tract after severe burn.J Trauma Acute Care Surg. 2017 Oct;83(4):716-724. doi: 10.1097/TA.0000000000001624. J Trauma Acute Care Surg. 2017. PMID: 28930963
-
Ischemic postconditioning regulates cardiomyocyte autophagic activity following ischemia/reperfusion injury.Mol Med Rep. 2015 Jul;12(1):1169-76. doi: 10.3892/mmr.2015.3533. Epub 2015 Mar 24. Mol Med Rep. 2015. PMID: 25816157
-
Autophagy in the stress-induced myocardium.Front Biosci (Elite Ed). 2012 Jan 1;4(6):2131-41. doi: 10.2741/e530. Front Biosci (Elite Ed). 2012. PMID: 22202025 Review.
Cited by
-
Regulation of macroautophagy in amiodarone-induced pulmonary fibrosis.J Pathol Clin Res. 2015 Jun 3;1(4):252-63. doi: 10.1002/cjp2.20. eCollection 2015 Oct. J Pathol Clin Res. 2015. PMID: 27499909 Free PMC article.
-
Pathological Responses of Cardiac Mitochondria to Burn Trauma.Int J Mol Sci. 2020 Sep 11;21(18):6655. doi: 10.3390/ijms21186655. Int J Mol Sci. 2020. PMID: 32932869 Free PMC article. Review.
-
Use of 1H-nuclear magnetic resonance to screen a set of biomarkers for monitoring metabolic disturbances in severe burn patients.Crit Care. 2014 Jul 24;18(4):R159. doi: 10.1186/cc13999. Crit Care. 2014. PMID: 25059459 Free PMC article. Clinical Trial.
-
H(+)/Cl(‑) exchange transporter 7 promotes lysosomal acidification‑mediated autophagy in mouse cardiomyocytes.Mol Med Rep. 2021 Mar;23(3):222. doi: 10.3892/mmr.2021.11861. Epub 2021 Jan 26. Mol Med Rep. 2021. PMID: 33495814 Free PMC article.
-
Hepatic autophagy after severe burn in response to endoplasmic reticulum stress.J Surg Res. 2014 Mar;187(1):128-33. doi: 10.1016/j.jss.2013.09.042. Epub 2013 Oct 2. J Surg Res. 2014. PMID: 24209807 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

