Activated human CD4+CD45RO+ memory T-cells indirectly inhibit NLRP3 inflammasome activation through downregulation of P2X7R signalling
- PMID: 22768094
- PMCID: PMC3387029
- DOI: 10.1371/journal.pone.0039576
Activated human CD4+CD45RO+ memory T-cells indirectly inhibit NLRP3 inflammasome activation through downregulation of P2X7R signalling
Abstract
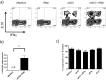
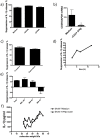
Inflammasomes are multi-protein complexes that control the production of pro-inflammatory cytokines such as IL-1β. Inflammasomes play an important role in the control of immunity to tumors and infections, and also in autoimmune diseases, but the mechanisms controlling the activation of human inflammasomes are largely unknown. We found that human activated CD4+CD45RO+ memory T-cells specifically suppress P2X7R-mediated NLRP3 inflammasome activation, without affecting P2X7R-independent NLRP3 or NLRP1 inflammasome activation. The concomitant increase in pro-IL-1β production induced by activated memory T-cells concealed this effect. Priming with IFNβ decreased pro-IL-1β production in addition to NLRP3 inflammasome inhibition and thus unmasked the inhibitory effect on NLRP3 inflammasome activation. IFNβ suppresses NLRP3 inflammasome activation through an indirect mechanism involving decreased P2X7R signaling. The inhibition of pro-IL-1β production and suppression of NLRP3 inflammasome activation by IFNβ-primed human CD4+CD45RO+ memory T-cells is partly mediated by soluble FasL and is associated with down-regulated P2X7R mRNA expression and reduced response to ATP in monocytes. CD4+CD45RO+ memory T-cells from multiple sclerosis (MS) patients showed a reduced ability to suppress NLRP3 inflammasome activation, however their suppressive ability was recovered following in vivo treatment with IFNβ. Thus, our data demonstrate that human P2X7R-mediated NLRP3 inflammasome activation is regulated by activated CD4+CD45RO+ memory T cells, and provide new information on the mechanisms mediating the therapeutic effects of IFNβ in MS.
Conflict of interest statement
Figures





References
-
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. - PubMed
-
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010. pp. 821–832. - PubMed
-
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004. pp. 319–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

