Workflow in clinical trial sites & its association with near miss events for data quality: ethnographic, workflow & systems simulation
- PMID: 22768105
- PMCID: PMC3387261
- DOI: 10.1371/journal.pone.0039671
Workflow in clinical trial sites & its association with near miss events for data quality: ethnographic, workflow & systems simulation
Abstract
Background: With the exponential expansion of clinical trials conducted in (Brazil, Russia, India, and China) and VISTA (Vietnam, Indonesia, South Africa, Turkey, and Argentina) countries, corresponding gains in cost and enrolment efficiency quickly outpace the consonant metrics in traditional countries in North America and European Union. However, questions still remain regarding the quality of data being collected in these countries. We used ethnographic, mapping and computer simulation studies to identify/address areas of threat to near miss events for data quality in two cancer trial sites in Brazil.
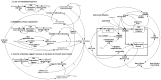
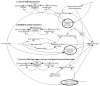
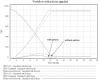
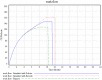
Methodology/principal findings: Two sites in Sao Paolo and Rio Janeiro were evaluated using ethnographic observations of workflow during subject enrolment and data collection. Emerging themes related to threats to near miss events for data quality were derived from observations. They were then transformed into workflows using UML-AD and modeled using System Dynamics. 139 tasks were observed and mapped through the ethnographic study. The UML-AD detected four major activities in the workflow evaluation of potential research subjects prior to signature of informed consent, visit to obtain subject́s informed consent, regular data collection sessions following study protocol and closure of study protocol for a given project. Field observations pointed to three major emerging themes: (a) lack of standardized process for data registration at source document, (b) multiplicity of data repositories and (c) scarcity of decision support systems at the point of research intervention. Simulation with policy model demonstrates a reduction of the rework problem.
Conclusions/significance: Patterns of threats to data quality at the two sites were similar to the threats reported in the literature for American sites. The clinical trial site managers need to reorganize staff workflow by using information technology more efficiently, establish new standard procedures and manage professionals to reduce near miss events and save time/cost. Clinical trial sponsors should improve relevant support systems.
Conflict of interest statement
Figures

Similar articles
-
Standardizing clinical trials workflow representation in UML for international site comparison.PLoS One. 2010 Nov 9;5(11):e13893. doi: 10.1371/journal.pone.0013893. PLoS One. 2010. PMID: 21085484 Free PMC article.
-
Extension of the primary care research object model (PCROM) as clinical research information model (CRIM) for the "learning healthcare system".BMC Med Inform Decis Mak. 2014 Dec 18;14:118. doi: 10.1186/s12911-014-0118-2. BMC Med Inform Decis Mak. 2014. PMID: 25519481 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Ethnography in Nutrition and Dietetics Research: A Systematic Review.J Acad Nutr Diet. 2018 Oct;118(10):1903-1942.e10. doi: 10.1016/j.jand.2018.06.002. Epub 2018 Aug 20. J Acad Nutr Diet. 2018. PMID: 30139629
-
The Minderoo-Monaco Commission on Plastics and Human Health.Ann Glob Health. 2023 Mar 21;89(1):23. doi: 10.5334/aogh.4056. eCollection 2023. Ann Glob Health. 2023. PMID: 36969097 Free PMC article. Review.
Cited by
-
Cancer clinical research in Latin America: current situation and opportunities. Expert opinion from the first ESMO workshop on clinical trials, Lima, 2015.ESMO Open. 2016 Jun 17;1(4):e000055. doi: 10.1136/esmoopen-2016-000055. eCollection 2016. ESMO Open. 2016. PMID: 27843620 Free PMC article. Review.
-
Detecting dissonance in clinical and research workflow for translational psychiatric registries.PLoS One. 2013 Sep 20;8(9):e75167. doi: 10.1371/journal.pone.0075167. eCollection 2013. PLoS One. 2013. PMID: 24073246 Free PMC article.
-
Glocal clinical registries: pacemaker registry design and implementation for global and local integration--methodology and case study.PLoS One. 2013 Jul 25;8(7):e71090. doi: 10.1371/journal.pone.0071090. Print 2013. PLoS One. 2013. PMID: 23936257 Free PMC article.
-
Applied Practice and Possible Leverage Points for Information Technology Support for Patient Screening in Clinical Trials: Qualitative Study.JMIR Med Inform. 2020 Jun 16;8(6):e15749. doi: 10.2196/15749. JMIR Med Inform. 2020. PMID: 32442156 Free PMC article.
-
Simplifying electronic data capture in clinical trials: workflow embedded image and biosignal file integration and analysis via web services.J Digit Imaging. 2014 Oct;27(5):571-80. doi: 10.1007/s10278-014-9694-z. J Digit Imaging. 2014. PMID: 24802371 Free PMC article.
References
-
- Normile D. The promise and pitfalls of clinical trials overseas. Science 322: 214–216. 2008. doi:10.1126/science.322.5899.214. - PubMed
-
- Thiers FA, Sinskey AJ, Berndt ER. Trends in the globalization of clinical trials. Nature Reviews Drug Discovery 7: 13–14. 2008. doi:10.1038/nrd2441.
-
- Dwivedi A, Bali RK, Wickramasinghe N, Naguib RNG. How workflow management systems enable the achievement of value driven healthcare delivery. Int J Electron Healthc. 2007;3:382–393. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

