Scaling-up of dental pulp stem cells isolated from multiple niches
- PMID: 22768154
- PMCID: PMC3387222
- DOI: 10.1371/journal.pone.0039885
Scaling-up of dental pulp stem cells isolated from multiple niches
Abstract
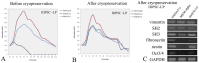
Dental pulp (DP) can be extracted from child's primary teeth (deciduous), whose loss occurs spontaneously by about 5 to 12 years. Thus, DP presents an easy accessible source of stem cells without ethical concerns. Substantial quantities of stem cells of an excellent quality and at early (2-5) passages are necessary for clinical use, which currently is a problem for use of adult stem cells. Herein, DPs were cultured generating stem cells at least during six months through multiple mechanical transfers into a new culture dish every 3-4 days. We compared stem cells isolated from the same DP before (early population, EP) and six months after several mechanical transfers (late population, LP). No changes, in both EP and LP, were observed in morphology, expression of stem cells markers (nestin, vimentin, fibronectin, SH2, SH3 and Oct3/4), chondrogenic and myogenic differentiation potential, even after cryopreservation. Six hours after DP extraction and in vitro plating, rare 5-bromo-2'-deoxyuridine (BrdU) positive cells were observed in pulp central part. After 72 hours, BrdU positive cells increased in number and were found in DP periphery, thus originating a multicellular population of stem cells of high purity. Multiple stem cell niches were identified in different zones of DP, because abundant expression of nestin, vimentin and Oct3/4 proteins was observed, while STRO-1 protein localization was restricted to perivascular niche. Our finding is of importance for the future of stem cell therapies, providing scaling-up of stem cells at early passages with minimum risk of losing their "stemness".
Conflict of interest statement
Figures





References
-
- Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

