Enterococcus faecium stimulates human neutrophils via the formyl-peptide receptor 2
- PMID: 22768166
- PMCID: PMC3386911
- DOI: 10.1371/journal.pone.0039910
Enterococcus faecium stimulates human neutrophils via the formyl-peptide receptor 2
Abstract
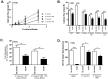
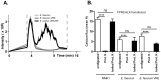
The human formyl-peptide receptor 2 (FPR2/ALX) senses phenol-soluble modulin (PSM) peptide toxins produced by pathogenic staphylococcal species and plays a crucial role in directing neutrophil influx during staphylococcal infection. However, it has remained unclear if FPR2 responds also to molecules from other bacterial pathogens. Here we analyzed a variety of gram-positive and gram-negative pathogens and found that apart from staphylococci only certain enterococcal strains have the capacity to stimulate FPR2/ALX. Most of the analyzed Enterococcus faecium but only sporadic Enterococcus faecalis strains released FPR2/ALX-stimulating molecules leading to neutrophil calcium ion fluxes, chemotaxis, and complement receptor upregulation. Among ten test strains vancomycin-resistant E. faecium had a significantly higher capacity to stimulate FPR2/ALX than vancomycin-susceptible strains, suggesting an association of strong FPR2/ALX activation with health-care associated strains. The enterococcal FPR2/ALX agonists were found to be peptides or proteins, which appear, however, to be unrelated to staphylococcal PSMs in sequence and physicochemical properties. Enterococci are among the most frequent invasive bacterial pathogens but the basis of enterococcal virulence and immune activation has remained incompletely understood. Our study indicates that previously unrecognized proteinaceous agonists contribute to Enterococcus-host interaction and underscores the importance of FPR2/ALX in host defense against major endogenous bacterial pathogens.
Conflict of interest statement
Figures


References
-
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. - PubMed
-
- Marasco WA, Phan SH, Krutzsch H, Showell HJ, Feltner DE, et al. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984;259:5430–5439. - PubMed
-
- Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

