Cardiac kallikrein-kinin system is upregulated in chronic volume overload and mediates an inflammatory induced collagen loss
- PMID: 22768235
- PMCID: PMC3387019
- DOI: 10.1371/journal.pone.0040110
Cardiac kallikrein-kinin system is upregulated in chronic volume overload and mediates an inflammatory induced collagen loss
Abstract
Background: The clinical problem of a "pure volume overload" as in isolated mitral or aortic regurgitation currently has no documented medical therapy that attenuates collagen loss and the resultant left ventricular (LV) dilatation and failure. Here, we identify a potential mechanism related to upregulation of the kallikrein-kinin system in the volume overload of aortocaval fistula (ACF) in the rat.
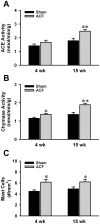
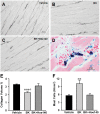
Methodology/principal findings: LV interstitial fluid (ISF) collection, hemodynamics, and echocardiography were performed in age-matched shams and 4 and 15 wk ACF rats. ACF rats had LV dilatation and a 2-fold increase in LV end-diastolic pressure, along with increases in LV ISF bradykinin, myocardial kallikrein and bradykinin type-2 receptor (BK(2)R) mRNA expression. Mast cell numbers were increased and interstitial collagen was decreased at 4 and 15 wk ACF, despite increases in LV ACE and chymase activities. Treatment with the kallikrein inhibitor aprotinin preserved interstitial collagen, prevented the increase in mast cells, and improved LV systolic function at 4 wk ACF. To establish a cause and effect between ISF bradykinin and mast cell-mediated collagen loss, direct LV interstitial bradykinin infusion in vivo for 24 hrs produced a 2-fold increase in mast cell numbers and a 30% decrease in interstitial collagen, which were prevented by BK(2)R antagonist. To further connect myocardial stretch with cellular kallikrein-kinin system upregulation, 24 hrs cyclic stretch of adult cardiomyocytes and fibroblasts produced increased kallikrein, BK(2)R mRNA expressions, bradykinin protein and gelatinase activity, which were all decreased by the kallikrein inhibitor-aprotinin.
Conclusions/significance: A pure volume overload is associated with upregulation of the kallikrein-kinin system and ISF bradykinin, which mediates mast cell infiltration, extracellular matrix loss, and LV dysfunction-all of which are improved by kallikrein inhibition. The current investigation provides important new insights into future potential medical therapies for the volume overload of aortic and mitral regurgitation.
Conflict of interest statement
Figures





References
-
- Borer JS, Bonow RO. Contemporary approach to aortic and mitral regurgitation. Circulation. 2003;108:2432–2438. - PubMed
-
- Chandrashekhar Y. Embracing the diversity of remodeling. J Am Coll Cardol. 2007;49:822–825. - PubMed
-
- Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006;48:e1–e148. - PubMed
-
- Ryan TD, Rothstein EC, Aban I, Tallaj JA, Husain A, et al. Left ventricular eccentric remodeling and matrix loss are mediated by bradykinin and precede cardiomyocyte elongation in rats with volume overload. J Am Coll Cardiol. 2007;49:811–821. - PubMed
-
- Wei CC, Lucchesi PA, Tallaj J, Bradley WE, Powell PC, et al. Cardiac interstitial bradykinin and mast cells modulate pattern of LV remodeling in volume overload in rats. Am J Physiol Heart Circ Physiol. 2003;285:H784–H792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

