Ell3 enhances differentiation of mouse embryonic stem cells by regulating epithelial-mesenchymal transition and apoptosis
- PMID: 22768269
- PMCID: PMC3386972
- DOI: 10.1371/journal.pone.0040293
Ell3 enhances differentiation of mouse embryonic stem cells by regulating epithelial-mesenchymal transition and apoptosis
Abstract
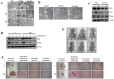
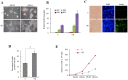
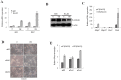
Ell3 is a testis-specific RNA polymerase II elongation factor whose cellular function is not clear. The present study shows that Ell3 is activated during the differentiation of mouse embryonic stem cells (mESCs). Furthermore, Ell3 plays a critical role in stimulating lineage differentiation of mESCs by promoting epithelial-mesenchymal transition (EMT) and suppressing apoptosis. Mouse ESCs engineered to stably express Ell3 were rapidly differentiated compared with control cells either under spontaneous differentiation or neural lineage-specific differentiation conditions. Gene expression profile and quantitative RT-PCR analysis showed that the expression of EMT markers, such as Zeb1 and Zeb2, two major genes that regulate EMT, was upregulated in Ell3-overexpressing mESCs. Remarkably, knockdown of Zeb1 attenuated the enhanced differentiation capacity of Ell3-overexpressing mESCs, which indicates that Ell3 plays a role in the induction of mESC differentiation by inducing EMT. In contrast to Ell3-overexpressing mESCs, Ell3-knock down mESCs could not differentiate under differentiation conditions and, instead, underwent caspase-dependent apoptosis. In addition, apoptosis of differentiating Ell3-knock out mESCs was associated with enhanced expression of p53. The present results suggest that Ell3 promotes the differentiation of mESCs by activating the expression of EMT-related genes and by suppressing p53 expression.
Conflict of interest statement
Figures


References
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
-
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
-
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

