Pharmacokinetics of quinacrine efflux from mouse brain via the P-glycoprotein efflux transporter
- PMID: 22768295
- PMCID: PMC3388068
- DOI: 10.1371/journal.pone.0039112
Pharmacokinetics of quinacrine efflux from mouse brain via the P-glycoprotein efflux transporter
Abstract
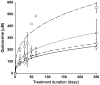
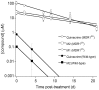
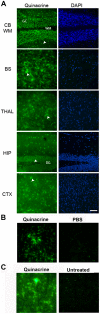
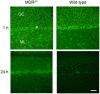
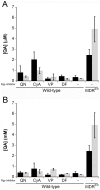
The lipophilic cationic compound quinacrine has been used as an antimalarial drug for over 75 years but its pharmacokinetic profile is limited. Here, we report on the pharmacokinetic properties of quinacrine in mice. Following an oral dose of 40 mg/kg/day for 30 days, quinacrine concentration in the brain of wild-type mice was maintained at a concentration of ∼1 µM. As a substrate of the P-glycoprotein (P-gp) efflux transporter, quinacrine is actively exported from the brain, preventing its accumulation to levels that may show efficacy in some disease models. In the brains of P-gp-deficient Mdr1(0/0) mice, we found quinacrine reached concentrations of ∼80 µM without any signs of acute toxicity. Additionally, we examined the distribution and metabolism of quinacrine in the wild-type and Mdr1(0/0) brains. In wild-type mice, the co-administration of cyclosporin A, a known P-gp inhibitor, resulted in a 6-fold increase in the accumulation of quinacrine in the brain. Our findings argue that the inhibition of the P-gp efflux transporter should improve the poor pharmacokinetic properties of quinacrine in the CNS.
Conflict of interest statement
Figures

References
-
- Collins SJ, Lewis V, Brazier M, Hill AF, Fletcher A, et al. Quinacrine does not prolong survival in a murine Creutzfeldt-Jakob disease model. Ann Neurol. 2002;52:503–506. - PubMed
-
- Benito-Leon J. Combined quinacrine and chlorpromazine therapy in fatal familial insomnia. Clin Neuropharmacol. 2004;27:201–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

