Triadin/Junctin double null mouse reveals a differential role for Triadin and Junctin in anchoring CASQ to the jSR and regulating Ca(2+) homeostasis
- PMID: 22768324
- PMCID: PMC3388061
- DOI: 10.1371/journal.pone.0039962
Triadin/Junctin double null mouse reveals a differential role for Triadin and Junctin in anchoring CASQ to the jSR and regulating Ca(2+) homeostasis
Abstract
Triadin (Tdn) and Junctin (Jct) are structurally related transmembrane proteins thought to be key mediators of structural and functional interactions between calsequestrin (CASQ) and ryanodine receptor (RyRs) at the junctional sarcoplasmic reticulum (jSR). However, the specific contribution of each protein to the jSR architecture and to excitation-contraction (e-c) coupling has not been fully established. Here, using mouse models lacking either Tdn (Tdn-null), Jct (Jct-null) or both (Tdn/Jct-null), we identify Tdn as the main component of periodically located anchors connecting CASQ to the RyR-bearing jSR membrane. Both proteins proved to be important for the structural organization of jSR cisternae and retention of CASQ within them, but with different degrees of impact. Our results also suggest that the presence of CASQ is responsible for the wide lumen of the jSR cisternae. Using Ca(2+) imaging and Ca(2+) selective microelectrodes we found that changes in e-c coupling, SR Ca(2+)content and resting [Ca(2+)] in Jct, Tdn and Tdn/Jct-null muscles are directly correlated to the effect of each deletion on CASQ content and its organization within the jSR. These data suggest that in skeletal muscle the disruption of Tdn/CASQ link has a more profound effect on jSR architecture and myoplasmic Ca(2+) regulation than Jct/CASQ association.
Conflict of interest statement
Figures
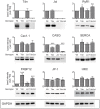
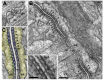
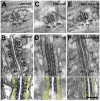
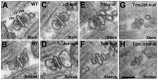


Similar articles
-
Junctin and triadin each activate skeletal ryanodine receptors but junctin alone mediates functional interactions with calsequestrin.Int J Biochem Cell Biol. 2009 Nov;41(11):2214-24. doi: 10.1016/j.biocel.2009.04.017. Epub 2009 May 4. Int J Biochem Cell Biol. 2009. PMID: 19398037 Free PMC article.
-
The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium.Biophys J. 2004 Apr;86(4):2121-8. doi: 10.1016/S0006-3495(04)74271-X. Biophys J. 2004. PMID: 15041652 Free PMC article.
-
Triadins modulate intracellular Ca(2+) homeostasis but are not essential for excitation-contraction coupling in skeletal muscle.J Biol Chem. 2007 Dec 28;282(52):37864-74. doi: 10.1074/jbc.M705702200. Epub 2007 Nov 2. J Biol Chem. 2007. PMID: 17981799
-
Control of muscle ryanodine receptor calcium release channels by proteins in the sarcoplasmic reticulum lumen.Clin Exp Pharmacol Physiol. 2009 Mar;36(3):340-5. doi: 10.1111/j.1440-1681.2008.05094.x. Clin Exp Pharmacol Physiol. 2009. PMID: 19278523 Review.
-
Calsequestrin and the calcium release channel of skeletal and cardiac muscle.Prog Biophys Mol Biol. 2004 May;85(1):33-69. doi: 10.1016/j.pbiomolbio.2003.07.001. Prog Biophys Mol Biol. 2004. PMID: 15050380 Review.
Cited by
-
A new cytoplasmic interaction between junctin and ryanodine receptor Ca2+ release channels.J Cell Sci. 2015 Mar 1;128(5):951-63. doi: 10.1242/jcs.160689. Epub 2015 Jan 20. J Cell Sci. 2015. PMID: 25609705 Free PMC article.
-
Quantification of the calcium signaling deficit in muscles devoid of triadin.PLoS One. 2022 Feb 25;17(2):e0264146. doi: 10.1371/journal.pone.0264146. eCollection 2022. PLoS One. 2022. PMID: 35213584 Free PMC article.
-
Myoplasmic resting Ca2+ regulation by ryanodine receptors is under the control of a novel Ca2+-binding region of the receptor.Biochem J. 2014 Jun 1;460(2):261-71. doi: 10.1042/BJ20131553. Biochem J. 2014. PMID: 24635445 Free PMC article.
-
Organization of junctional sarcoplasmic reticulum proteins in skeletal muscle fibers.J Muscle Res Cell Motil. 2015 Dec;36(6):501-15. doi: 10.1007/s10974-015-9421-5. Epub 2015 Sep 15. J Muscle Res Cell Motil. 2015. PMID: 26374336 Review.
-
The Structural-Functional Crosstalk of the Calsequestrin System: Insights and Pathological Implications.Biomolecules. 2023 Nov 23;13(12):1693. doi: 10.3390/biom13121693. Biomolecules. 2023. PMID: 38136565 Free PMC article. Review.
References
-
- Jones LR, Zhang L, Sanborn K, Jorgensen AO, Kelley J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J Biol Chem. 1995;270:30787–30796. - PubMed
-
- Caswell AH, Brandt NR, Brunschwig JP, Purkerson S. Localization and partial characterization of the oligomeric disulfide-linked molecular weight 95,000 protein (triadin) which binds the ryanodine and dihydropyridine receptors in skeletal muscle triadic vesicles. Biochemistry. 1991;30:7507–7513. - PubMed
-
- Knudson CM, Stang KK, Moomaw CR, Slaughter CA, Campbell KP. Primary structure and topological analysis of a skeletal muscle-specific junctional sarcoplasmic reticulum glycoprotein (triadin). J Biol Chem. 1993;268:12646–12654. - PubMed
-
- Meissner G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1975;389:51–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 AR054818/AR/NIAMS NIH HHS/United States
- 5K01AR054818/AR/NIAMS NIH HHS/United States
- R01 AR055104/AR/NIAMS NIH HHS/United States
- HL64018/HL/NHLBI NIH HHS/United States
- R03 AR047605/AR/NIAMS NIH HHS/United States
- P01 AR052354/AR/NIAMS NIH HHS/United States
- P01AR052354/AR/NIAMS NIH HHS/United States
- R01 HL064018/HL/NHLBI NIH HHS/United States
- P01AR47605/AR/NIAMS NIH HHS/United States
- HL26057/HL/NHLBI NIH HHS/United States
- R37 HL026057/HL/NHLBI NIH HHS/United States
- AR055104/AR/NIAMS NIH HHS/United States
- R01 HL026057/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

