A pro-cathepsin L mutant is a luminal substrate for endoplasmic-reticulum-associated degradation in C. elegans
- PMID: 22768338
- PMCID: PMC3388072
- DOI: 10.1371/journal.pone.0040145
A pro-cathepsin L mutant is a luminal substrate for endoplasmic-reticulum-associated degradation in C. elegans
Abstract
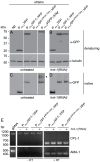
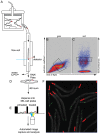
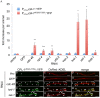
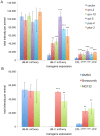
Endoplasmic-reticulum associated degradation (ERAD) is a major cellular misfolded protein disposal pathway that is well conserved from yeast to mammals. In yeast, a mutant of carboxypeptidase Y (CPY*) was found to be a luminal ER substrate and has served as a useful marker to help identify modifiers of the ERAD pathway. Due to its ease of genetic manipulation and the ability to conduct a genome wide screen for modifiers of molecular pathways, C. elegans has become one of the preferred metazoans for studying cell biological processes, such as ERAD. However, a marker of ERAD activity comparable to CPY* has not been developed for this model system. We describe a mutant of pro-cathepsin L fused to YFP that no longer targets to the lysosome, but is efficiently eliminated by the ERAD pathway. Using this mutant pro-cathepsin L, we found that components of the mammalian ERAD system that participate in the degradation of ER luminal substrates were conserved in C. elegans. This transgenic line will facilitate high-throughput genetic or pharmacological screens for ERAD modifiers using widefield epifluorescence microscopy.
Conflict of interest statement
Figures

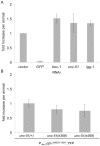
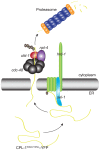
References
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. - PubMed
-
- Hoseki J, Ushioda R, Nagata K. Mechanism and components of endoplasmic reticulum-associated degradation. J Biochem. 2010;147:19–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

