The role of self-monitoring of blood glucose in glucagon-like peptide-1-based treatment approaches: a European expert recommendation
- PMID: 22768899
- PMCID: PMC3440044
- DOI: 10.1177/193229681200600323
The role of self-monitoring of blood glucose in glucagon-like peptide-1-based treatment approaches: a European expert recommendation
Abstract
The role of glucagon-like peptide (GLP)-1-based treatment approaches for type 2 diabetes mellitus (T2DM) is increasing. Although self-monitoring of blood glucose (SMBG) has been performed in numerous studies on GLP-1 analogs and dipeptidyl peptidase-4 inhibitors, the potential role of SMBG in GLP-1-based treatment strategies has not been elaborated. The expert recommendation suggests individualized SMBG strategies in GLP-1-based treatment approaches and suggests simple and clinically applicable SMBG schemes. Potential benefits of SMBG in GLP-1-based treatment approaches are early assessment of treatment success or failure, timely modification of treatment, detection of hypoglycemic episodes, assessment of glucose excursions, and support of diabetes management and diabetes education. Its length and frequency should depend on the clinical setting and the quality of metabolic control. It is considered to play an important role for the optimization of diabetes management in T2DM patients treated with GLP-1-based approaches.
© 2012 Diabetes Technology Society.
Figures

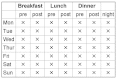
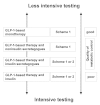
References
-
- Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13(1):7–18. - PubMed
-
- Deacon CF. Therapeutic strategies based on glucagon-like peptide 1. Diabetes. 2004;53(9):2181–2189. - PubMed
-
- Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36(8):741–744. - PubMed
-
- Frias JP, Edelman SV. Incretins and their role in the management of diabetes. Curr Opin Endocrinol Diabetes Obes. 2007;14(4):269–276. - PubMed
-
- Schnell O, Alawi H, Battelino T, Ceriello A, Diem P, Felton A, Grzeszczak W, Harno K, Kempler P, Satman I, Vergès B. Addressing schemes of self-monitoring of blood glucose in type 2 diabetes: a European perspective and expert recommendation. Diabetes Technol Ther. 2011;13(9):959–965. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

