Efficacy of Olibra: a 12-week randomized controlled trial and a review of earlier studies
- PMID: 22768902
- PMCID: PMC3440037
- DOI: 10.1177/193229681200600326
Efficacy of Olibra: a 12-week randomized controlled trial and a review of earlier studies
Abstract
Background: Intervention strategies that harness the body's appetite and satiety regulating signals provide a means of countering excessive energy intake.
Methods: Eighty-two subjects were enrolled (18-60 years, body mass index: 25-40 kg/m(2)) in a randomized, placebo-controlled, double-blind, parallel trial. During a 12-week period, the effects of Olibra™ fat emulsion (2.1 g twice daily) on food intake, appetite, satiety, weight, and body composition were compared with those of a twice daily administered placebo (1.95 g milk fat). On days -7, 0, and 28, Olibra or the placebo added to 200 g of yogurt was served at breakfast and lunch. Food intake, appetite, and satiety were assessed after lunch and dinner. Body weight was measured on days -7, 0, 14, 28, 56, and 84. Body fat, waist circumference, and waist-hip ratio were determined on days 0 and 84. The Eating Inventory was administered at screening and on day 28. Data relating to 71 subjects were analyzed using analysis of covariance.
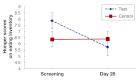
Results: At 12 weeks, body weight was reduced in the test group (2.17 ± 0.46 kg standard error of the mean, p < .0001) and the control group (1.68 ± 0.42 kg, p < .0001). Waist circumference decreased by 2.93 ± 0.85 cm in the test group (p = .001) and by 1.78 ± 0.74 cm in the control group (p = .02). Differential weight and waist circumference reductions were not significant. Hunger scores (Eating Inventory) decreased more in the test group (p = .0082). Differential group effects were not significant for body fat, waist-hip ratio, food intake, appetite, and satiety.
Conclusions: At this dose, Olibra did not exert a consistent effect on food intake, appetite regulation, body weight, or body composition.
Trial registration: ClinicalTrials.gov NCT01416051.
© 2012 Diabetes Technology Society.
Figures
Comment in
-
An analysis of the "Effect of Olibra: a 12-week randomized control trial and a review of earlier studies".J Diabetes Sci Technol. 2012 May 1;6(3):709-11. doi: 10.1177/193229681200600327. J Diabetes Sci Technol. 2012. PMID: 22768903 Free PMC article.
Similar articles
-
An analysis of the "Effect of Olibra: a 12-week randomized control trial and a review of earlier studies".J Diabetes Sci Technol. 2012 May 1;6(3):709-11. doi: 10.1177/193229681200600327. J Diabetes Sci Technol. 2012. PMID: 22768903 Free PMC article.
-
Investigation of the medium-term effects of Olibratrade mark fat emulsion on food intake in non-obese subjects.Eur J Clin Nutr. 2006 Sep;60(9):1081-91. doi: 10.1038/sj.ejcn.1602422. Epub 2006 Mar 15. Eur J Clin Nutr. 2006. PMID: 16538239 Clinical Trial.
-
Long-term effects of consumption of a novel fat emulsion in relation to body-weight management.Int J Obes (Lond). 2007 Jun;31(6):942-9. doi: 10.1038/sj.ijo.0803532. Epub 2007 Feb 13. Int J Obes (Lond). 2007. PMID: 17299383 Clinical Trial.
-
Appetite-Suppressing and Satiety-Increasing Bioactive Phytochemicals: A Systematic Review.Nutrients. 2019 Sep 17;11(9):2238. doi: 10.3390/nu11092238. Nutrients. 2019. PMID: 31533291 Free PMC article.
-
Proteins and Peptides from Food Sources with Effect on Satiety and Their Role as Anti-Obesity Agents: A Narrative Review.Nutrients. 2024 Oct 20;16(20):3560. doi: 10.3390/nu16203560. Nutrients. 2024. PMID: 39458554 Free PMC article. Review.
Cited by
-
An analysis of the "Effect of Olibra: a 12-week randomized control trial and a review of earlier studies".J Diabetes Sci Technol. 2012 May 1;6(3):709-11. doi: 10.1177/193229681200600327. J Diabetes Sci Technol. 2012. PMID: 22768903 Free PMC article.
-
Effect of macronutrient composition on short-term food intake and weight loss.Adv Nutr. 2015 May 15;6(3):302S-8S. doi: 10.3945/an.114.006957. Print 2015 May. Adv Nutr. 2015. PMID: 25979503 Free PMC article. Review.
References
-
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults. 1999–2008. JAMA. 2010;303(3):235–241. - PubMed
-
- Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. - PubMed
-
- Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16(10):2323–2330. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical